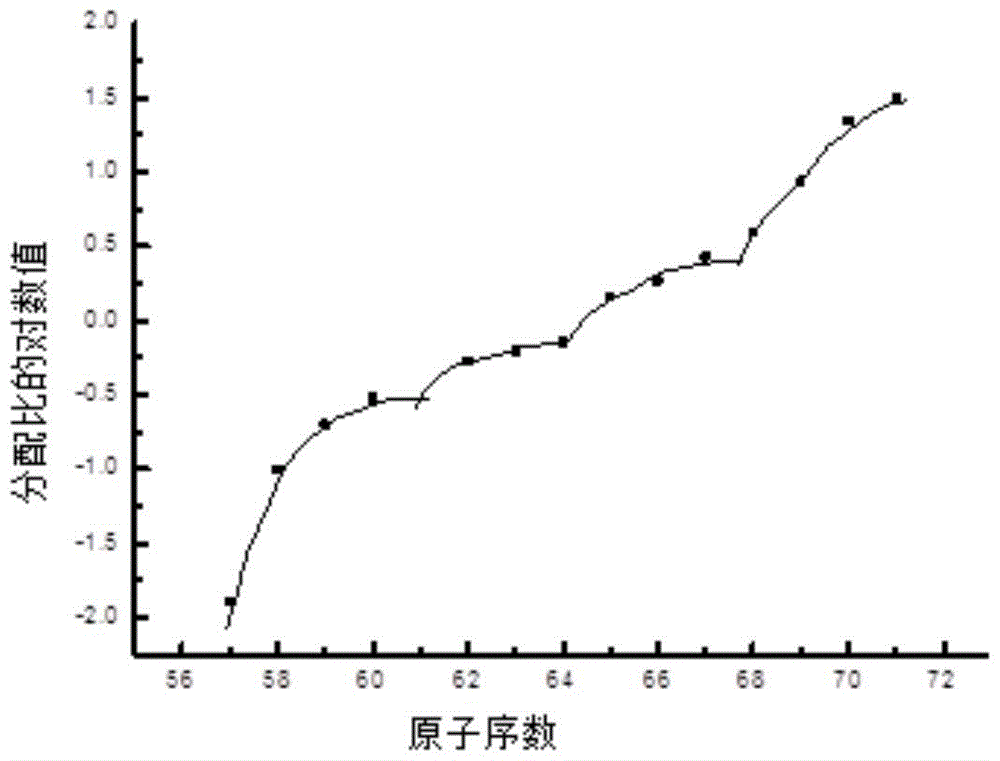
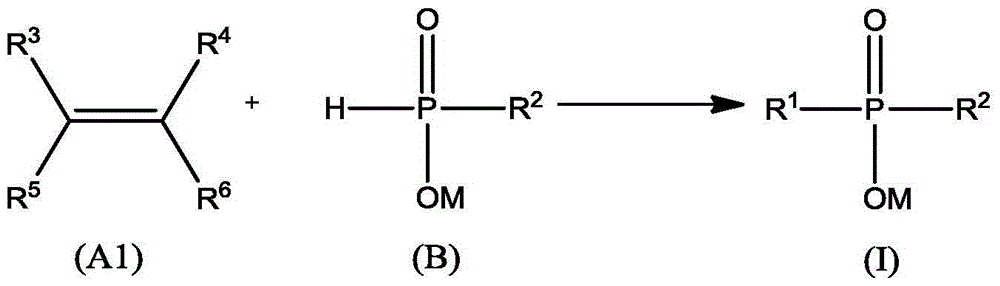
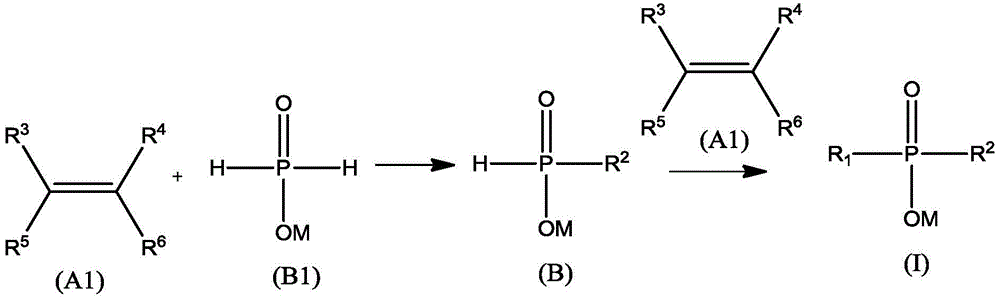
Preparation method and application of a kind of dialkylphosphinic acid compound or its salt
A technology of alkylphosphinic acid and compound, which is applied in the field of preparation of dialkylphosphinic acid compounds or their salts, and can solve problems such as harsh reaction conditions, many side reactions, and long reaction time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1 2
[0099] The synthesis of embodiment 1 two (n-octyl) phosphinic acid:
[0100]
[0101] Sodium hypophosphite monohydrate 10.6g (0.1mol), glacial acetic acid 15mL (0.26mol), N,N-dimethylformamide (DMF) 80mL, 1-octene 22.4g (0.2mol), azobisiso 3.3g (0.02mol) of butyronitrile was reacted at 130°C for 1h. After adding 3.3 g (0.02 mol) of the initiator azobisisobutyronitrile, the reaction was continued at 130° C. for 1 h. After the reaction, cool down, wash the reaction liquid twice with water, wash once with saturated saline, add 8% (wt) NaOH solution to wash, add 20% (wt) sulfuric acid solution to acidify, extract twice with ether, combine the organic phases, and wash with water After washing, it was dried over anhydrous sodium sulfate, filtered with suction, and the solvent was removed under reduced pressure to obtain 23.4 g of the product with a yield of 80.7%.
[0102] 31 P NMR: Di(n-octyl)phosphinic acid: δ: 59.7ppm, 98.3% (purity, by peak value)
[0103] Neutralization ...
Embodiment 2
[0104] The synthesis of embodiment 2 two (2,4,4-trimethylpentyl) phosphinic acid
[0105]
[0106] Sodium hypophosphite monohydrate 10.6g (0.1mol), glacial acetic acid 10mL (0.175mol), 2,4,4-trimethyl-1-pentene 30g (0.27mol), benzoyl peroxide 4.8g (0.02 mol), DMF80mL were added to the microwave synthesis reactor in sequence, heated to 135°C under microwave conditions, and reacted for 2h. After adding 4.8 g (0.02 mol) of initiator benzoyl peroxide, the reaction was continued at 135°C for 2 h, and the above process was repeated 3 times (0.1 mol of initiator was added during the entire reaction process). After the reaction, cool down, wash the reaction solution twice with water, once with saturated saline, add 8% (wt) NaOH solution for washing, add 20% (wt) hydrochloric acid solution to acidify, extract twice with ether, combine the organic phases, and wash with water After washing, it was dried over anhydrous sodium sulfate, filtered with suction, and the solvent was removed...
Embodiment 3
[0109] The synthesis of two (3,3-dimethylbutyl) phosphinic acid of embodiment 3
[0110]
[0111] Sodium hypophosphite monohydrate 10.6g (0.1mol), glacial acetic acid 5mL (0.089mol), ethylene glycol monomethyl ether 75mL, 3,3-dimethyl-1-butene 16.8g (0.2mol), peroxidized 4 mL (0.021 mol) of tert-butyl benzoate was sequentially added to a microwave synthesis reactor, and heated to 90°C for 1 h under microwave conditions. After adding 4 mL of initiator tert-butyl peroxybenzoate, the reaction was continued at 90° C. for 1 h. Wash twice with water, once with saturated saline, add 8% (wt) NaOH solution to wash, add 20% (wt) sulfuric acid solution to acidify, extract twice with ether, combine organic phases, wash with water, and dry over anhydrous sodium sulfate. Suction filtration and desolventization under reduced pressure gave 17.5 g of the product with a yield of 74.8%.
[0112] 31 P NMR: bis(3,3-dimethylbutyl) phosphinic acid: δ: 61.7ppm, 95.6% (purity, by peak value)
...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com