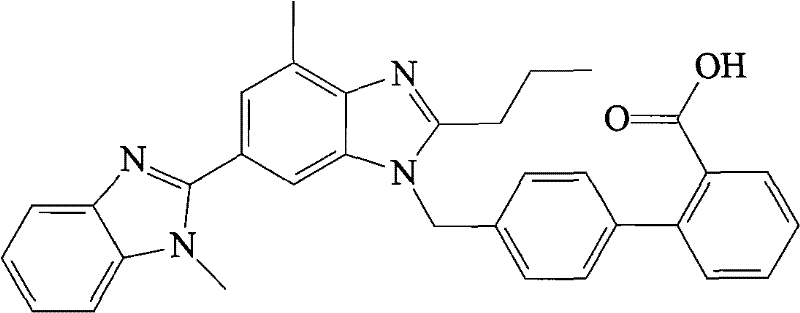
Telmisartan liposome solid preparation
A technology of telmisartan and solid preparations, which is applied in the field of pharmaceutical preparations, can solve problems such as uncontrollable drug release speed and drug release process, unsatisfactory long-term stability of samples, and hidden dangers in clinical use, so as to retain drug efficacy and improve Bioavailability, the effect of reducing toxic and side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
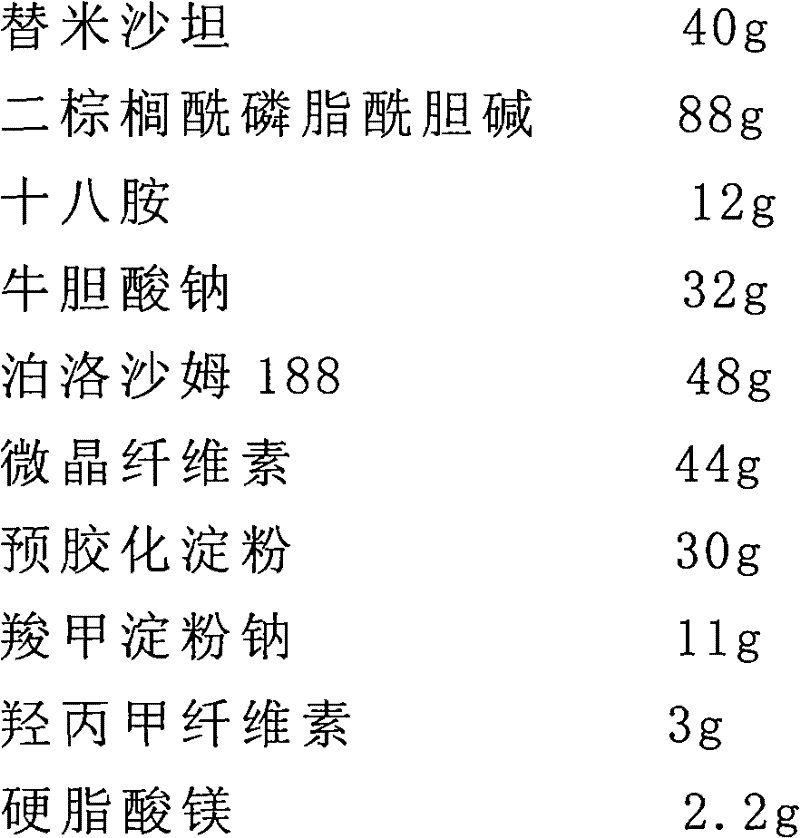
[0045] The preparation of embodiment 1 telmisartan liposome sheet
[0046] Prescription (1000 tablets)
[0047]
[0048] Preparation Process
[0049] (1) 40g telmisartan, 88g dipalmitoylphosphatidylcholine, 12g octadecylamine, 32g sodium taurocholate and 48g poloxamer 188 were dissolved in 2000ml volume ratio of 1:3 ethanol and isopropyl In a mixed solvent of ether, a lipid solution is obtained;
[0050] (2) Place the above-mentioned lipid solution in a pear-shaped bottle, and remove the mixed solvent by rotary evaporation in a constant temperature water bath at 50° C. to form a uniform lipid film;
[0051] (3) Prepare 1000 ml of citric acid-sodium citrate buffer solution with a pH value of 5.5, add it to a pear-shaped bottle and shake gently, so that the lipid film is eluted and dispersed in a hydration medium for dissolution, to obtain a liposome suspension;
[0052] (4) After the above-mentioned suspension is poured into a high-speed mixer and stirred evenly, filter w...
Embodiment 2
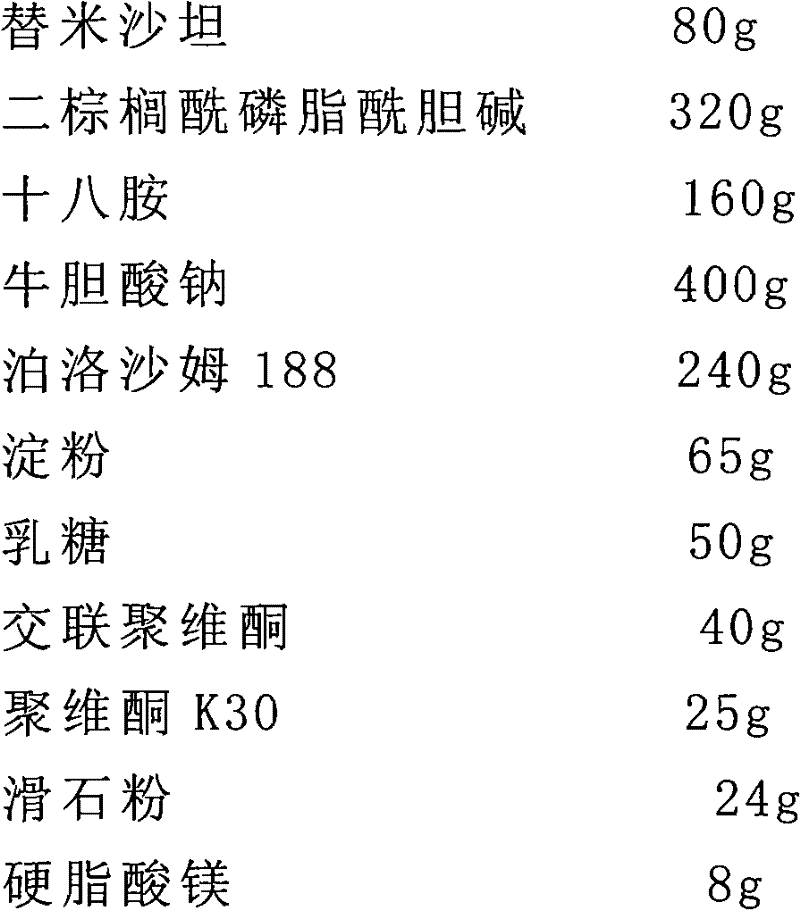
[0056] The preparation of embodiment 2 Telmisartan liposome sheet
[0057] Prescription (1000 tablets)
[0058]
[0059] Preparation Process
[0060] (1) 80g telmisartan, 320g dipalmitoylphosphatidylcholine, 160g octadecylamine, 400g sodium taurocholate and 240g poloxamer 188 were dissolved in 6000ml volume ratio of 1:3 ethanol and isopropyl In a mixed solvent of ether, a lipid solution is obtained;
[0061] (2) Place the above-mentioned lipid solution in a pear-shaped bottle, and remove the mixed solvent by rotary evaporation in a constant temperature water bath at 40° C. to form a uniform lipid film;
[0062] (3) Prepare 3000ml of potassium dihydrogen phosphate-dipotassium hydrogen phosphate buffer solution with a pH value of 5.5, add it to a pear-shaped bottle and shake gently, so that the lipid film is eluted and dispersed in a hydration medium for dissolution, to obtain a liposome suspension ;
[0063] (4) After the above-mentioned suspension is poured into a high-...
Embodiment 3
[0067] The preparation of embodiment 3 telmisartan liposome capsules
[0068] Prescription (1000 capsules)
[0069]
[0070] Preparation Process
[0071] (1) 40g telmisartan, 120g dipalmitoylphosphatidylcholine, 48g octadecylamine, 120g sodium taurocholate and 84g poloxamer 188 were dissolved in 2000ml volume ratio of 1:3 ethanol and isopropyl In a mixed solvent of ether, a lipid solution is obtained;
[0072] (2) Place the above-mentioned lipid solution in a pear-shaped bottle, and remove the mixed solvent by rotary evaporation in a constant temperature water bath at 45° C. to form a uniform lipid film;
[0073] (3) Prepare 1000 ml of acetic acid-sodium acetate buffer solution with a pH value of 5.5, add it to a pear-shaped bottle and shake gently, so that the lipid film is eluted and dispersed in a hydration medium for dissolving to obtain a liposome suspension;
[0074] (4) After the above-mentioned suspension is poured into a high-speed mixer and stirred evenly, filt...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com