A kind of preparation method of bis(2,4,6-trimethylbenzoyl) phenyl phosphine oxide
A technology of trimethylbenzoyl and phenylphosphine oxide, which is applied in the field of preparation of bisphenylphosphine oxide, can solve the problems of increasing by-products, unfavorable safety, failure to recycle, etc., and achieves the reduction of organic matter content and yield reduction. The effect of reducing the efficiency and reducing the process cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
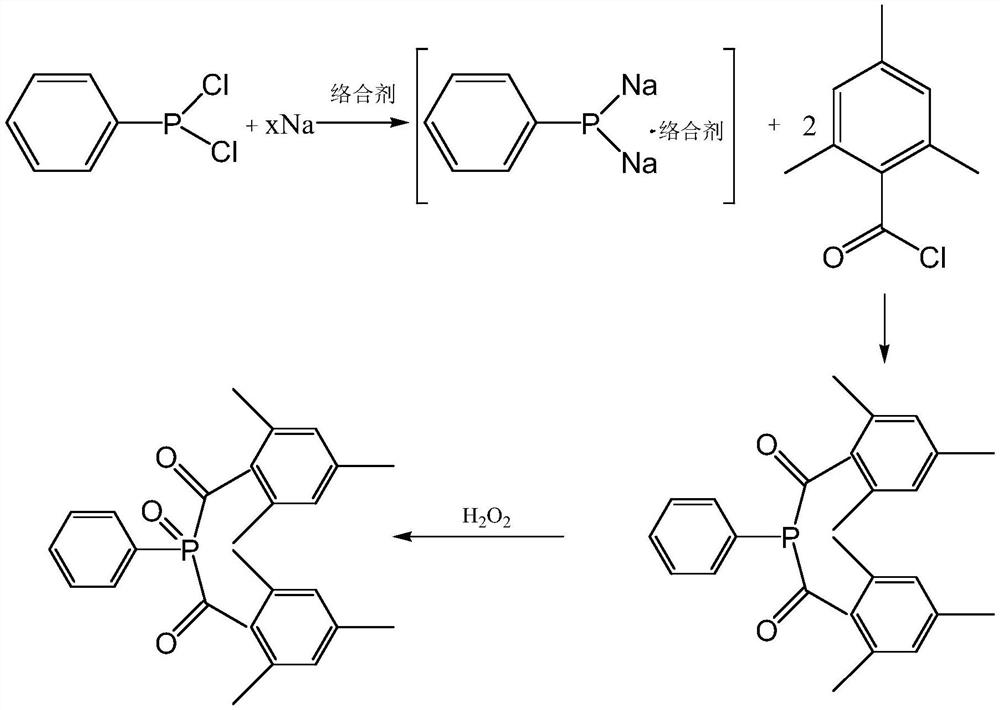
[0097] The present embodiment provides a preparation method of bis(2,4,6-trimethylbenzoyl)phenyl phosphine oxide, comprising:
[0098] (1) In a 500mL four-necked flask, add 11g of sodium metal and 150g of toluene, heat up to 100°C under nitrogen protection, and stir for 3h to beat the sodium metal into sodium sand to obtain a sodium sand suspension;
[0099] (2) keep the reflux state, in step (1), add 21g phenyl phosphine dichloride dropwise to the sodium-sand suspension, add about 2h, keep the reflux for 4h, until the reaction solution turns bright yellow, then under the reflux state 4.2g (0.5eq) of tetrahydrofuran was added, the dripping was completed in about 0.5h, the temperature was kept under reflux for 2h, the temperature was lowered to 70°C under nitrogen protection, 43g mes-trimethylbenzoyl chloride was added dropwise, the reaction temperature was controlled at 75°C, the addition was completed in about 2h, and 75°C was added dropwise. Incubate the reaction at ℃ for 8h...
Embodiment 2
[0104] The present embodiment provides a preparation method of bis(2,4,6-trimethylbenzoyl)phenyl phosphine oxide, comprising:
[0105] (1) In a 500mL four-neck flask, add 11g of sodium metal and 150g of toluene, add 4.2g (0.5eq) of tetrahydrofuran, heat up to 100°C under nitrogen protection, and stir for 3h to make sodium metal into sodium sand to obtain a sodium sand suspension ;
[0106] (2) maintain the reflux state, in step (1), add 21 g of phenyl phosphine dichloride dropwise to the sodium-sand suspension, add it for about 2 hours, and keep refluxing for 4 hours until the reaction solution turns bright yellow, and the nitrogen protection is lowered to 70°C, dropwise add 43g mes-trimethylbenzoyl chloride, control the reaction temperature to 75°C, finish the addition in about 2h, and keep the reaction at 75°C for 8h;
[0107] (3) After the reaction in step (2) is completed, add 120 g of water dropwise to the reaction solution, stir at room temperature for 0.5 h, stand for ...
Embodiment 3
[0112] The present embodiment provides a preparation method of bis(2,4,6-trimethylbenzoyl)phenyl phosphine oxide, comprising:
[0113] (1) In a 500mL four-neck flask, add 20g of metallic sodium and 150g of toluene, heat up to 100°C under nitrogen protection, and stir the sodium for 2h to make sodium sand to obtain a sodium sand suspension;
[0114] (2) keep the reflux state, add 21 g of phenylphosphine dichloride dropwise to the sodium-sand suspension, add it for about 2 h, keep it at reflux for 6 h, until the reaction solution becomes bright yellow, add 1.7 g of tetrahydrofuran (0.2 g of tetrahydrofuran (0.2 g) under reflux conditions eq), after dropping for about 0.5h, the temperature was kept under reflux for 4h, the temperature was lowered to 70°C under nitrogen protection, 43g of mesityl benzoyl chloride was added dropwise, the reaction temperature was controlled at 70°C, the addition was completed for about 2h, and the reaction was kept at 70°C for 10h;
[0115] (3) afte...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com