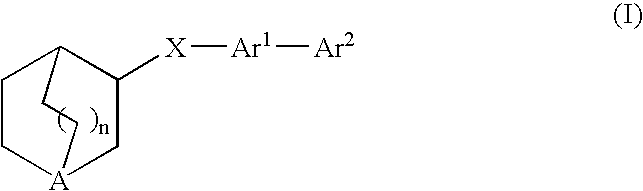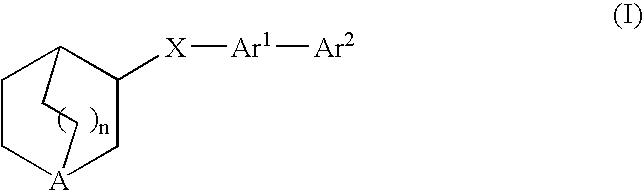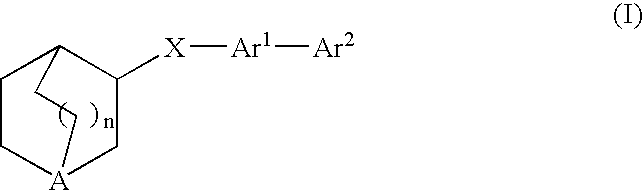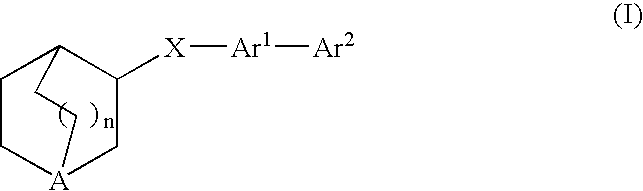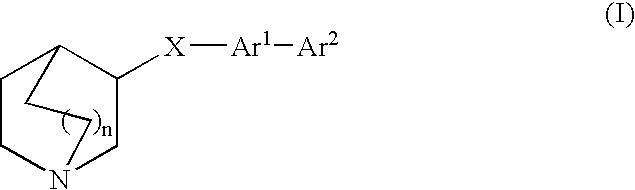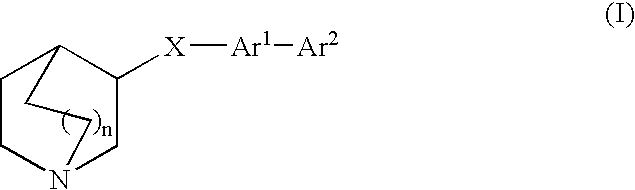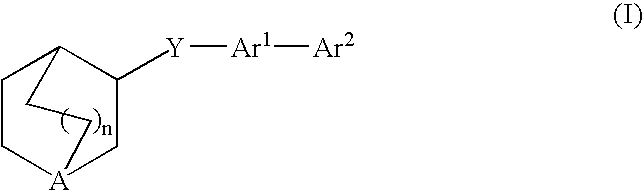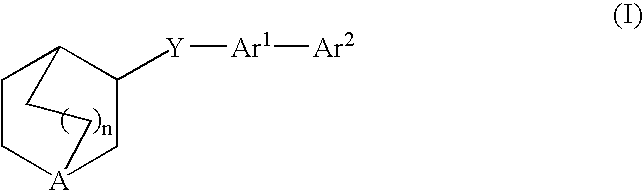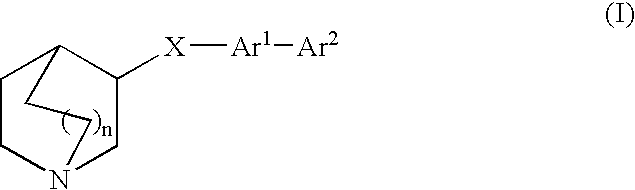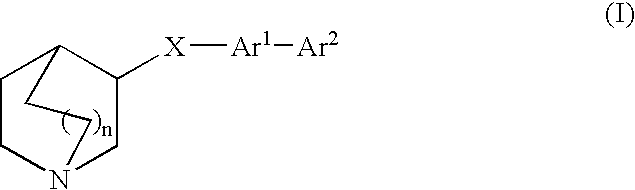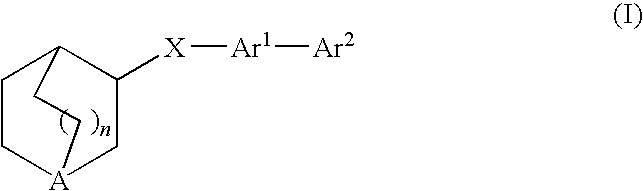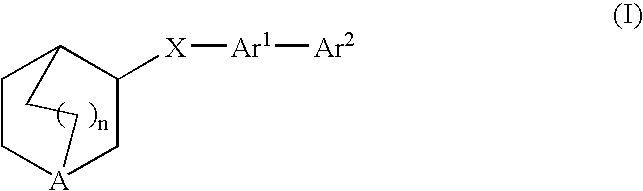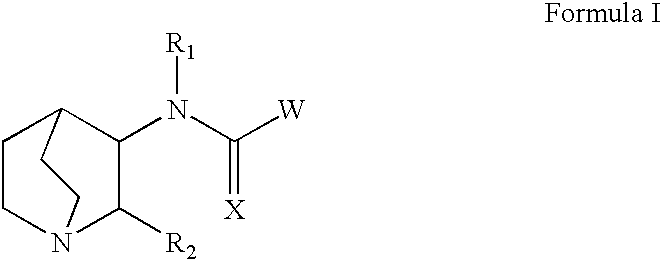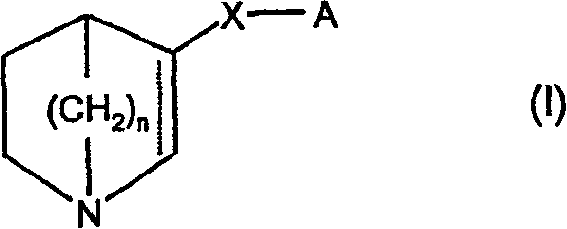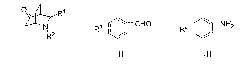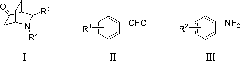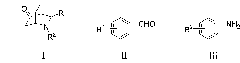Patents
Literature
119 results about "Quinuclidine" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Quinuclidine is an organic compound and a bicyclic amine and used as a catalyst and a chemical building block. It is a strong base with pKₐ of the conjugate acid of 11.0. It can be prepared by reduction of quinuclidone.
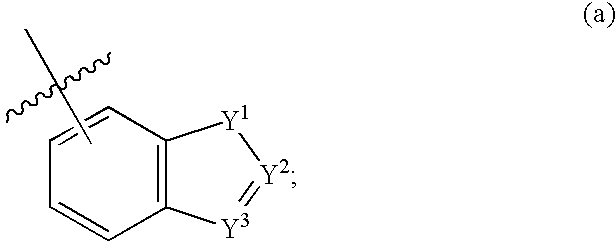
Fused bicycloheterocycle substituted quinuclidine derivatives
InactiveUS20050245531A1Improve cognitive functionLimited abilityBiocideNervous disorderQuinuclidineΑ7 nachr
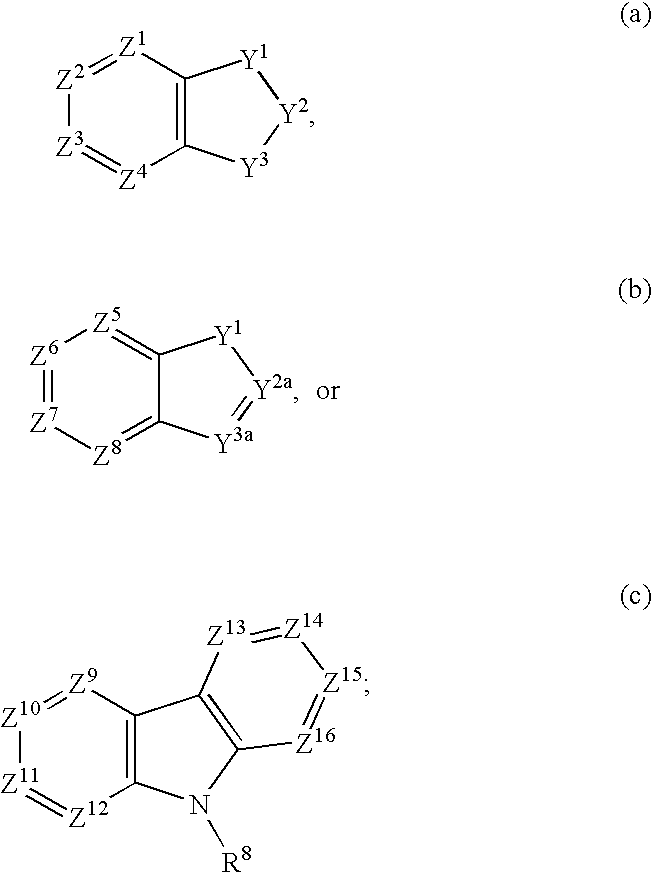
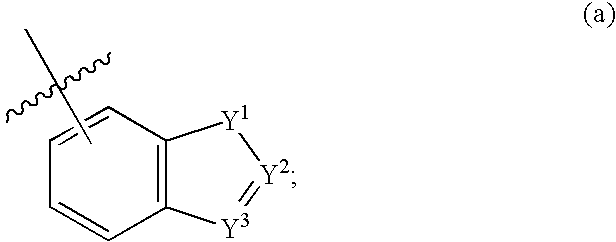
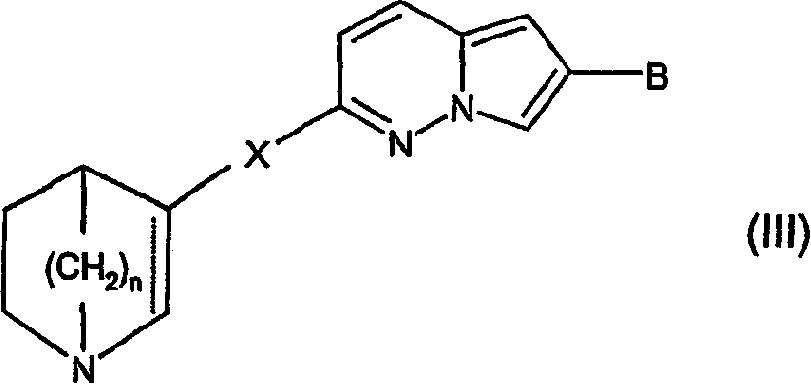
Compounds of formula (I) wherein n is 0, 1, or 2; A is N or N+—O—; X is O, S, —NH—, and —N-alkyl-; Ar1 is a 6-membered aromatic ring; and Ar2 is a fused bicycloheterocycle. The compounds are useful in treating conditions or disorders prevented by or ameliorated by α7 nAChR ligands. Also disclosed are pharmaceutical compositions having compounds of formula (I) and methods for using such compounds and compositions.
Owner:ABBOTT LAB INC
Fused bicycloheterocycle substituted quinuclidine derivatives
InactiveUS20050137204A1Improve cognitive functionLimited abilityBiocideOrganic chemistryQuinuclidineΑ7 nachr
Compounds of formula (I) wherein n is 0, 1, or 2; A is N or N+—O−; X is O, S, —NH—, and —N-alkyl-; Ar1 is a 6-membered aromatic ring; and Ar2 is a fused bicycloheterocycle. The compounds are useful in treating conditions or disorders prevented by or ameliorated by α7 nAChR ligands. Also disclosed are pharmaceutical compositions having compounds of formula (I) and methods for using such compounds and compositions.
Owner:ABBOTT LAB INC
Fused bicycloheterocycle substituted quinuclidine derivatives
ActiveUS7160876B2Improve cognitive functionLimited abilityBiocideNervous disorderQuinuclidineΑ7 nachr
Compounds of formula (I)wherein n is 0, 1, or 2; X is O, S, —NH—, and —N-alkyl-; Ar1 is a 6-membered aromatic ring; and Ar2 is a fused bicycloheterocycle. The compounds are useful in treating conditions or disorders prevented by or ameliorated by α7 nAChR ligands. Also disclosed are pharmaceutical compositions having compounds of formula (I) and methods for using such compounds and compositions.
Owner:ABBVIE INC
Quinuclidine compounds as alpha-7 nicotinic acetylcholine receptor ligands
The disclosure provides compounds of formula I, including their salts, as well as compositions and methods of using the compounds. The compounds are ligands for the nicotinic α7 receptor and may be useful for the treatment of various disorders of the central nervous system, especially affective and neurodegenerative disorders.
Owner:BRISTOL MYERS SQUIBB CO
3-Quinuclidinyl amino-substituted biaryl derivatives
InactiveUS20050159597A1Improve cognitive functionLimited abilityBiocideNervous disorderStereochemistryΑ7 nachr
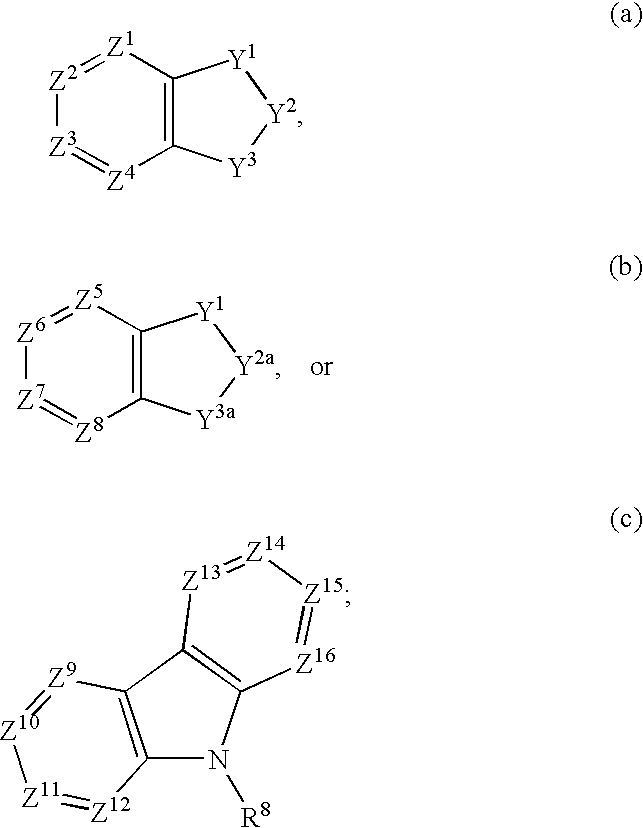
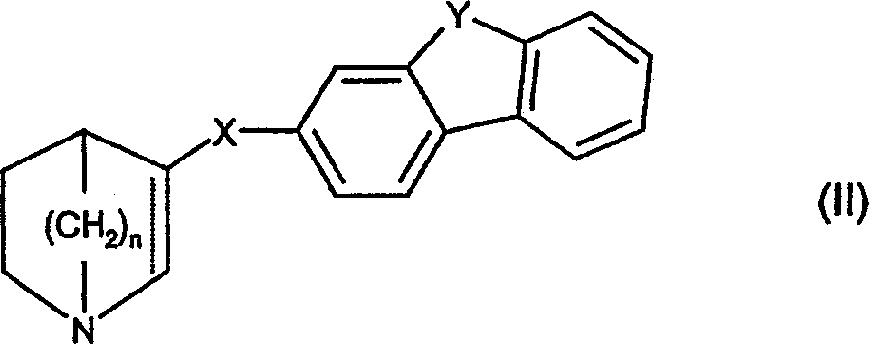
Compounds of formula (I) wherein A is N or N+—O−; n is 0, 1, or 2; Y is O, S, —NH—, and —N-alkyl-; Ar1 is both 6-membered aromatic rings; Ar2 is 5- or 6-membered aromatic rings with a —NR8R9 group, as defined herein. The compounds are useful in treating conditions or disorders prevented by or ameliorated by α7 nAChR ligands. Also disclosed are pharmaceutical compositions having compounds of formula (I) and methods for using such compounds and compositions.
Owner:ABBVIE INC
Fused bicycloheterocycle substituted quinuclidine derivatives
ActiveUS20050137184A1Improve cognitive functionLimited abilityBiocideNervous disorderQuinuclidineΑ7 nachr
Compounds of formula (I) wherein n is 0, 1, or 2; X is O, S, —NH—, and —N-alkyl-; Ar1 is a 6-membered aromatic ring; and Ar2 is a fused bicycloheterocycle. The compounds are useful in treating conditions or disorders prevented by or ameliorated by α7 nAChR ligands. Also disclosed are pharmaceutical compositions having compounds of formula (I) and methods for using such compounds and compositions.
Owner:ABBVIE INC
Agmatine, and polyaminoguanidine-bound heterocyclic compounds for neurotrauma and neurodegenerative diseases
InactiveUS6114392ABiocideIsocyanic acid derivatives preparationPhenothiazine derivativeDegenerative Disorder
The invention relates to the use of agmatine, in the treatment of acute neurotrauma (such as stroke) and degenerative disorders of the central and peripheral nervous system (such as dementia). The invention further provides novel compounds of general formula I (which are quinuclidine derivatives), formula II (which are norbornane derivatives), formula III (which are adamantane derivatives), and formula IV (which are phenothiazine derivatives): wherein R1, R2 and R3 are each independently hydrogen, hydroxy, substituted or unsubstituted C1-4 alkyl, substituted or unsubstituted C1-4 alkoxy, halogeno, amino, phenyl, or R4NR5; R4 and R5 are each independently hydrogen, or (CH2)n-[NH(CH2)x]y-NHR6, or (CH2)n-[NH(CH2)x]y-NH-NHR6, or (CH2)n-[NH(CH2)x]y-(NR7=)CNHR6, or (CH2)n-[NH(CH2)x]y-NH(NR7=)CNHR6 wherein n is from 0-5, y is from 0-5 and each x is independently from 1-5; R6, and R7 are each independently hydrogen, hydroxy, substituted or unsubstituted C1-4 alkyl, substituted or unsubstituted C1-4 alkoxy, or halogeno; and pharmaceutically acceptable salts and optically active isomers thereof.
Owner:GILAD GAD M +1
Fused bicycloheterocycle substituted quinuclidine derivatives
Owner:ABBVIE INC
Fused bicycloheterocycle substituted quinuclidine derivatives
InactiveUS20070060588A1Improve cognitive functionLimited abilityBiocideOrganic chemistryCombinatorial chemistryPerylene derivatives
Compounds of formula (I) wherein n is 0, 1, or 2; A is N or N+—O−; X is O, S, —NH—, and —N-alkyl-; Ar1 is a 6-membered aromatic ring; and Ar2 is a fused bicycloheterocycle. The compounds are useful in treating conditions or disorders prevented by or ameliorated by α7 nAChR ligands. Also disclosed are pharmaceutical compositions having compounds of formula (I) and methods for using such compounds and compositions.
Owner:ABBOTT LAB INC
Quinuclidines-substituted-multi-cyclic-heteroaryls for the treatment of disease
Owner:PHARMACIA & UPJOHN CO
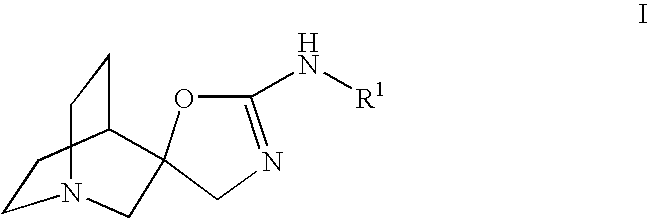
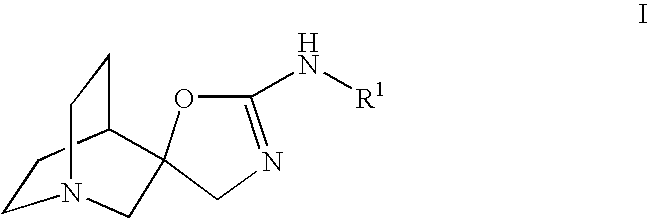
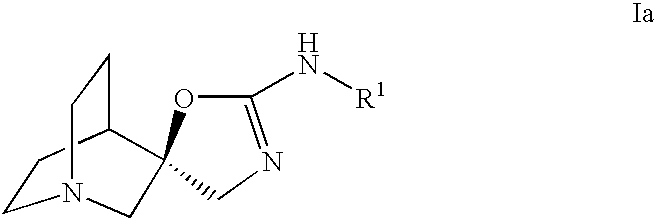
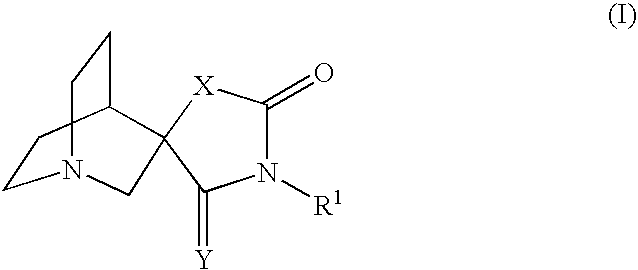
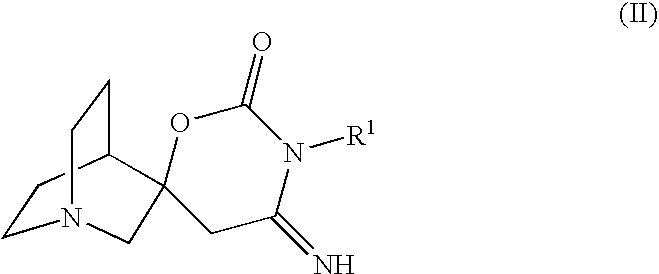
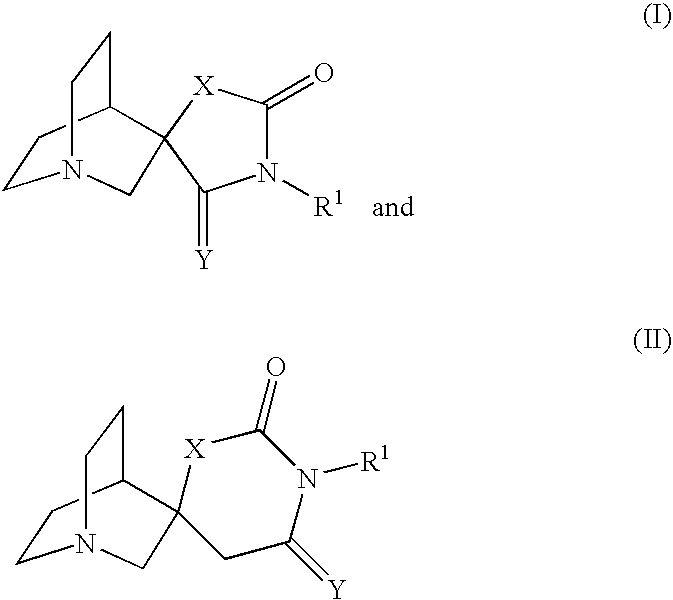
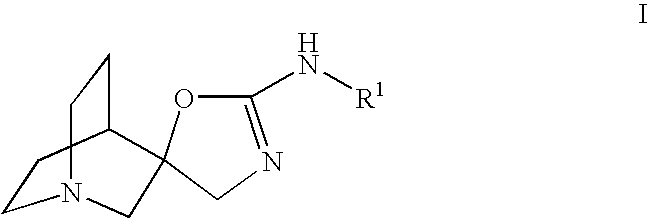
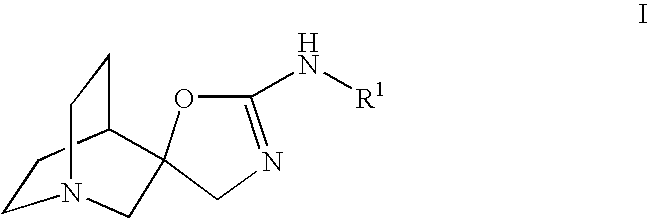
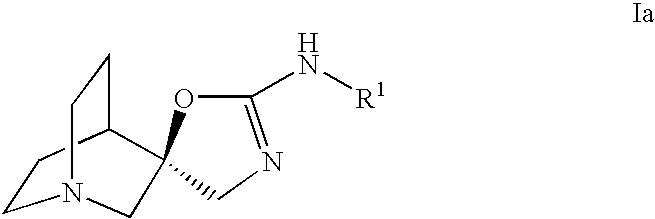
Novel spiro-quinuclidinyl derivatives for the treatment of central nervous system disorders
The present invention is directed to novel spiro-quinuclidinyl derivatives, pharmaceutical compositions containing them and their use in the treatment of central nervous system disorders.
Owner:JANSSEN PHARMA NV
Quinuclidine compounds as alpha-7 nicotinic acetylcholine receptor ligands
The disclosure provides compounds of formula I, including their salts, as well as compositions and methods of using the compounds. The compounds are ligands for the nicotinic α7 receptor and may be useful for the treatment of various disorders of the central nervous system, especially affective and neurodegenerative disorders.
Owner:BRISTOL MYERS SQUIBB CO
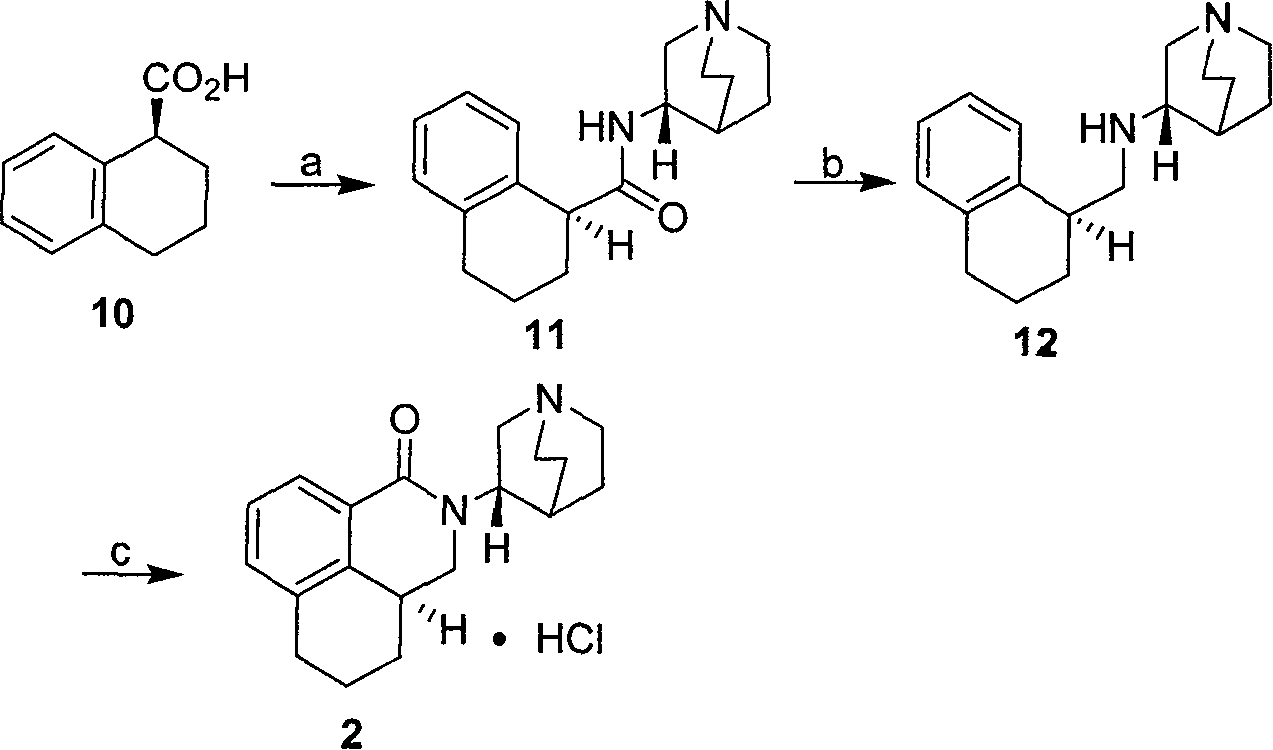
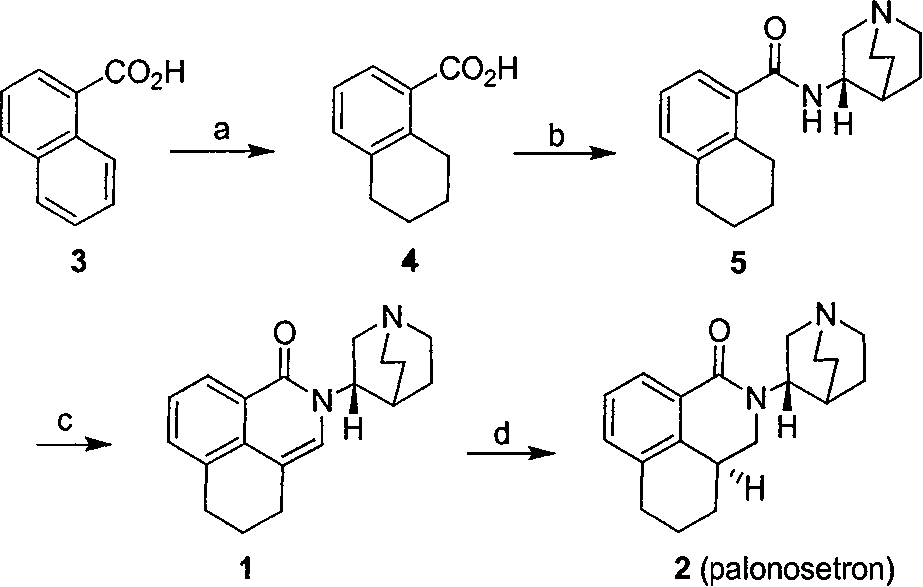
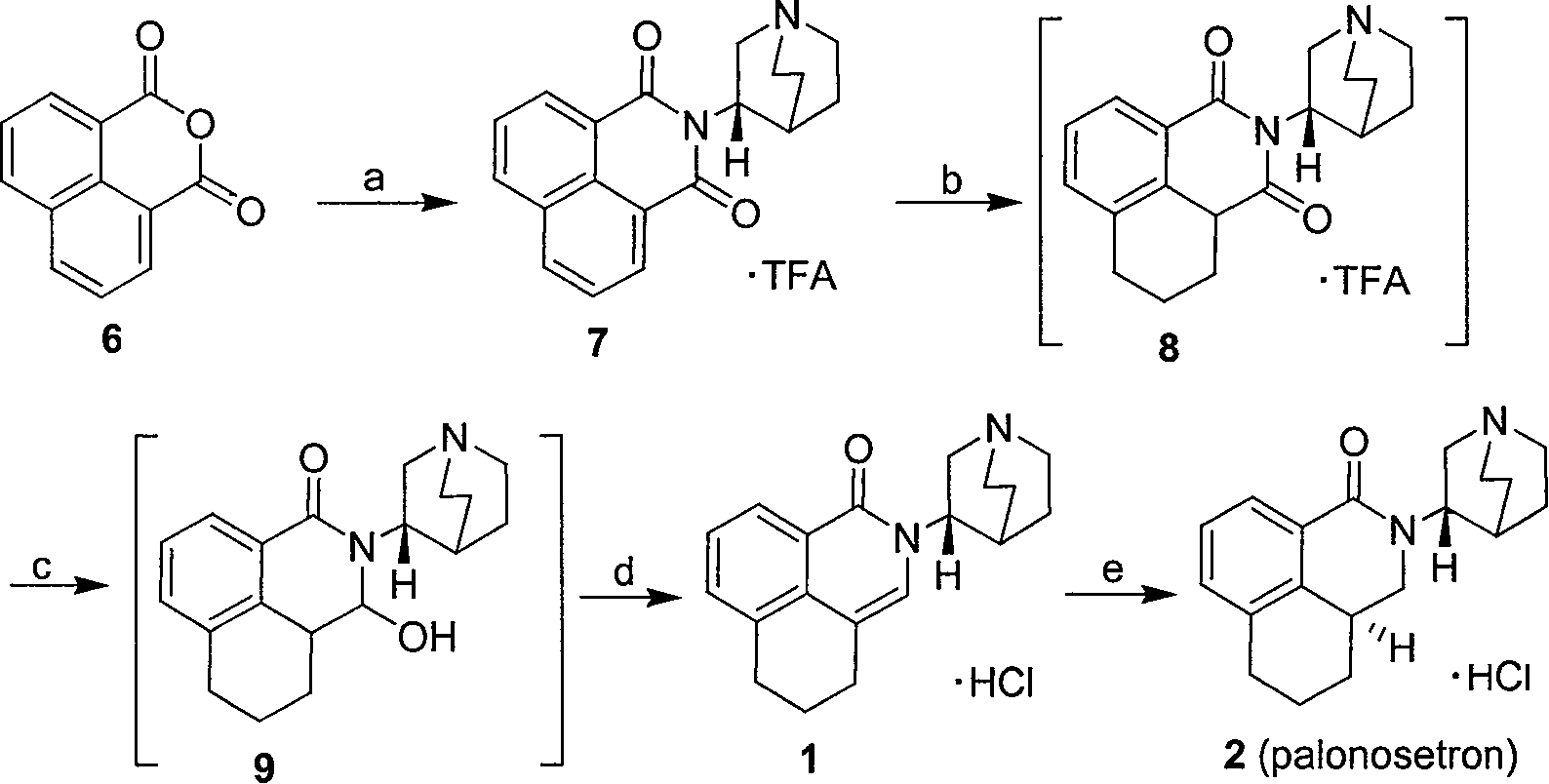
Method for synthesizing palonosetron hydrochloride
The invention discloses a novel synthesis method of palonosetron hydrochloride, which comprises that (1) (S)-tetralin formic acid is reacted with thionyl chloride and (S)-3-amido-quinine cyclic amine, to obtain (S, S)-quinuclidine tetralin formamide, (2), (S, S)-quinuclidine tetralin formamide is reacted with reductant and boron trifluoride diethyl etherate, to obtain (S, S)-tetralin methyl quinine cyclic amine, (3), (S, S)-tetralin methyl quinine cyclic amine is reacted with diphosgene to be added and reacted in boron trifluoride diethyl etherate solution, while the product is added and reacted with alcaine and water, to obtain palonosetron hydrochloride. And the synthesis route is represented as above: a: SOCI2, (S)-3-aminoquinuclidine, b: NaBH4, BF3OEt2, c: BF3OEt2, CICO2CCI3.
Owner:SHENZHEN NEPTUNUS PHARM CO LTD
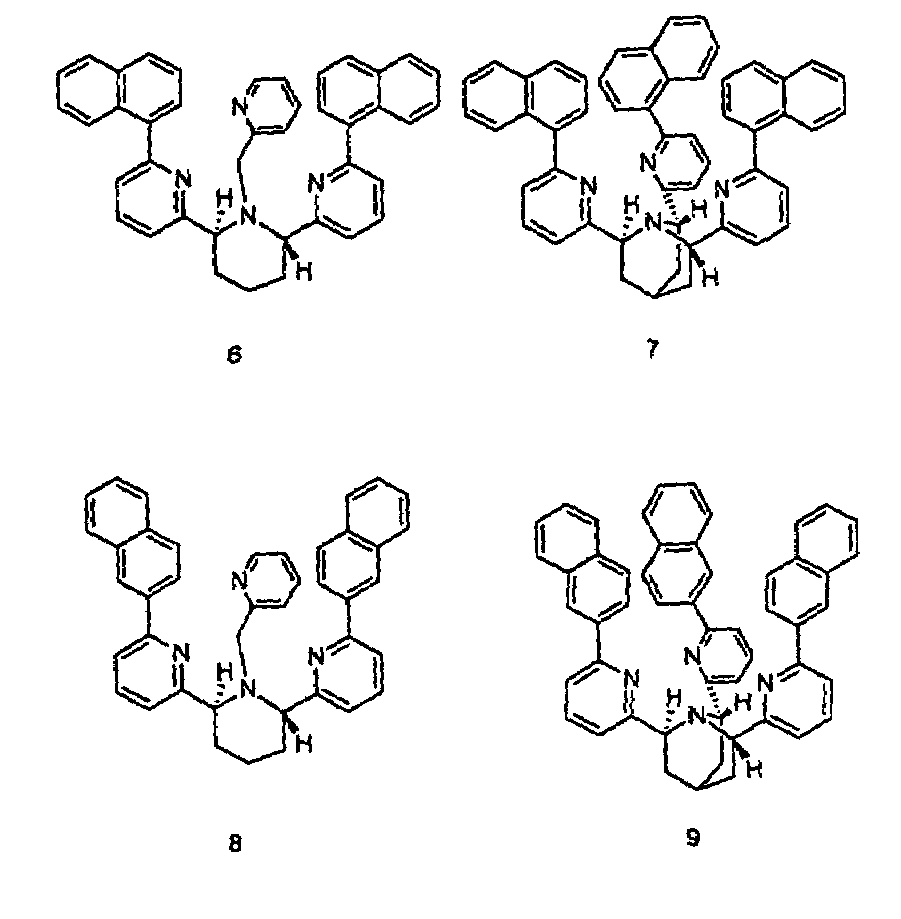
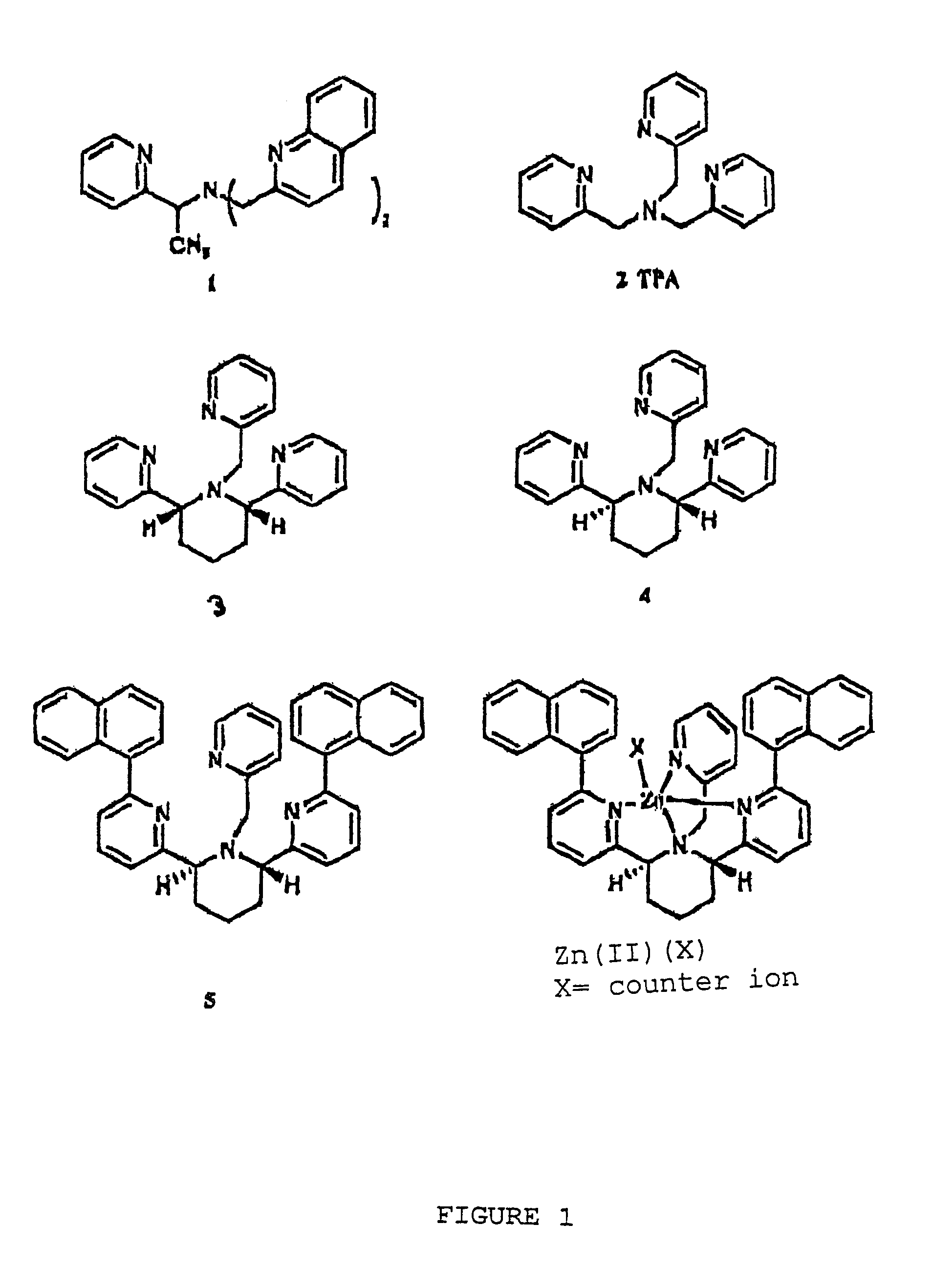
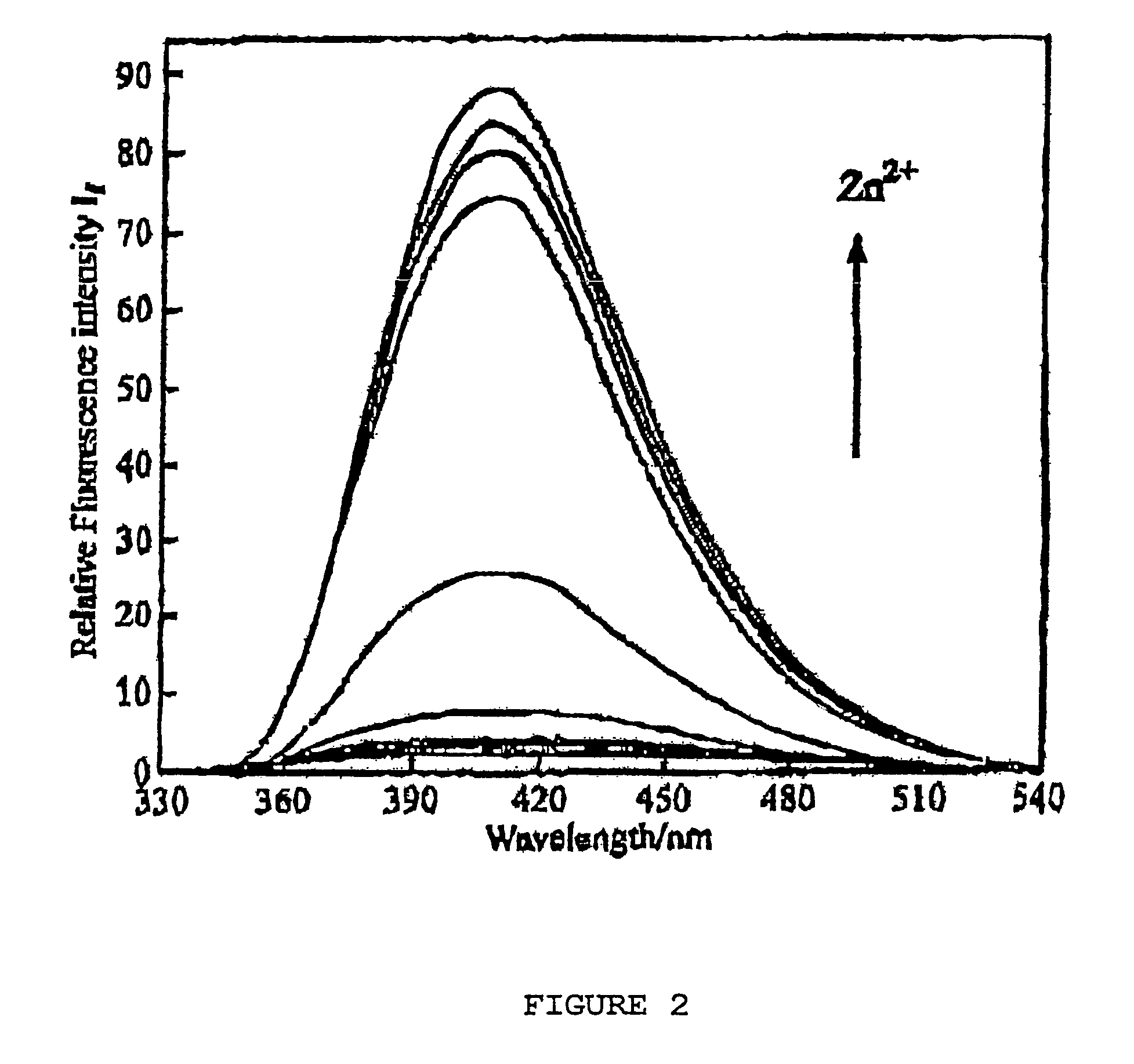
Chiral piperidine and quinucledine ligands
InactiveUS7491544B2High selectivityImprove rigidityAnalysis using chemical indicatorsChemiluminescene/bioluminescenceArylNaphthalene
Zn(II) is selectively detected in a sample by contacting the sample with a tripodal ligand with a piperidine or quinuclidine scaffold, one of which acts as a zinc sensor, in which the rigidity of the ligand scaffold is increased. The rigidity of the ligand scaffold can be increased by adding aromatic groups or cyclic hydrocarbon groups. Examples of aromatic groups include naphthalene and the like. Examples of cyclic groups include nitrogen-substituted cyclohexane and cyclohexene such as piperidine.
Owner:NEW YORK UNIV
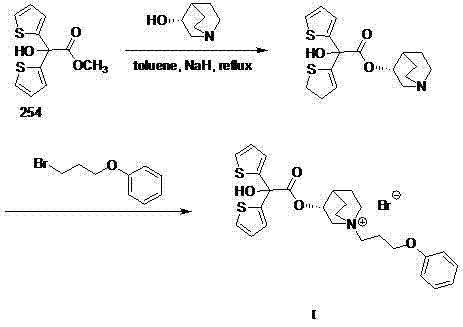
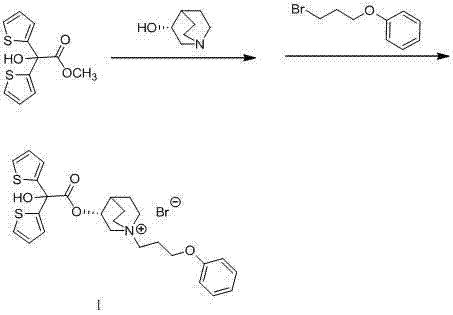
Technology for preparing aclidinium bromide employing one-pot process
The invention belongs to the field of medicinal chemistry, and discloses a technology for preparing aclidinium bromide. By adopting the technology, (R)-3-quinuclidinol, (C1)methyl-2,2-dithienyl glycolate and 3-phenoxy propyl bromide are taken as raw materials, and the aclidinium bromide is prepared by adopting a one-pot process. The method is simple to operate, and convenient for post-treatment, and has good application value.
Owner:AVENTIS PHARMA HAINAN
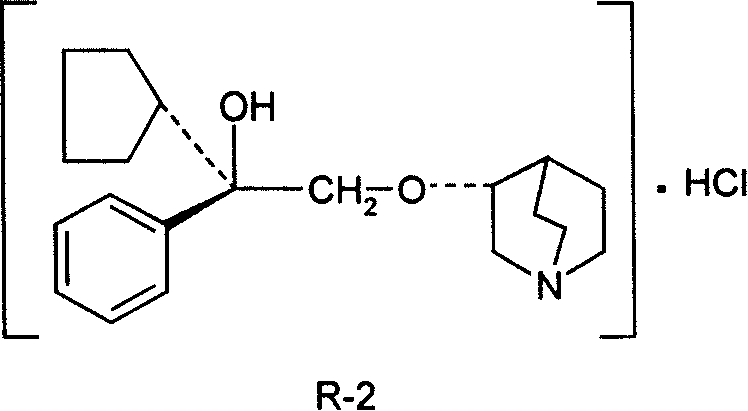
Pharmaceutical composition containing (3S, 2'R)-3-(2'-hydroxy-2'-cyclopentyl-2'-phenylethoxy) quinuclidine hydrochloride and uses thereof
InactiveCN101234108AHigh optical purityStereoselectiveOrganic active ingredientsUrinary disorderDiseaseSide effect
The invention discloses medical composition containing active component of (3S, 2'R)-3-(2'-hydroxyl-2'-cyclopentyl-2'-phenylethoxy) quinine quinuclidine chloride and application of the medical composition in preparing drugs for treating chronic obstructive lung disease, incontinentia urinae, diversified shock diseases and preparing adjuvant drugs used before anaesthesia. The active component of the medical composition of the invention has high optical purity, strong stereoselectivity in vivo process, definite curative effects and high pertinency to the disease, thus effectively avoiding generation of toxic and side effects.
Owner:CHENGDU LIST PHARMA
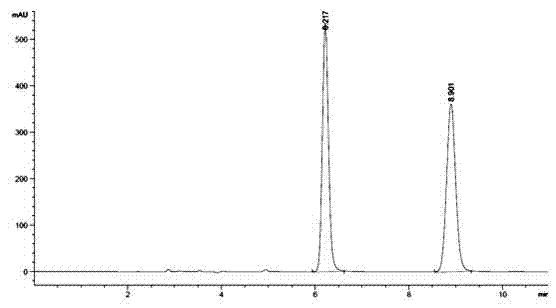
Method for measuring optical purity of (R)-3-quinuclidinol
ActiveCN104133018AEasy to identifyHigh purityComponent separationBenzenesulfinic acidMethyl palmoxirate
The invention belongs to the technical field of pharmaceutical chemical engineering, and in particular relates to a method for measuring the optical purity of (R)-3-quinuclidinol. The method for measuring the optical purity of (R)-3-quinuclidinol comprises the steps of (a) preparing a sample; (b) preparing a test solution; (c) preparing a chromatographic condition, and performing detection. According to the method, a precolumn derivatization chiral chromatography method is adopted to measure the optical purity of (R)-3-quinuclidinol; specifically, the precolumn derivatization method is used for enabling (RS)-3-quinuclidinol racemate and paratoluensulfonyl chloride to react to generate (RS)-3-quinuclidinol paratoluensulfonyl chloride derivative, namely quinuclidinol 3-paratoluene sulfonate; then the chiral chromatography method is used for measuring the optical purity; therefore, the technical difficulty in measurement of the optical purity of (R)-3-quinuclidinol is creatively solved.
Owner:JINAN ASIA PHARMA TECH
Preparation technology of solifenacin succinate
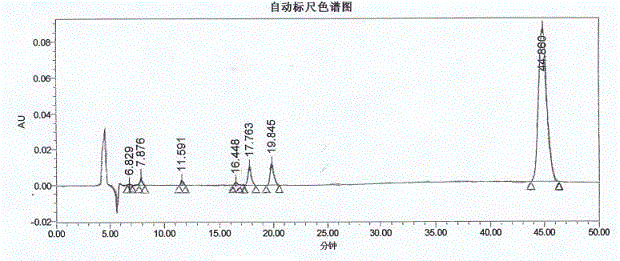
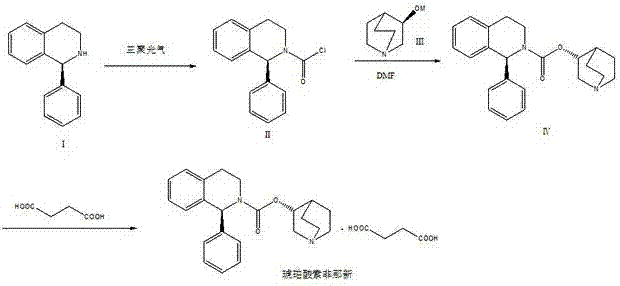
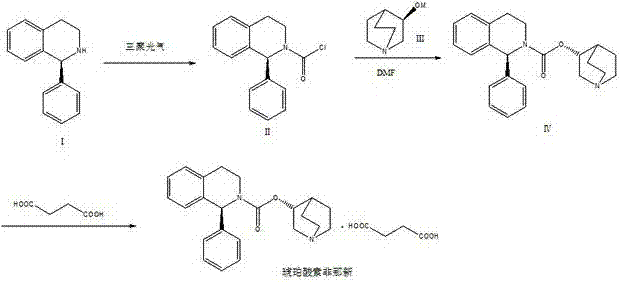
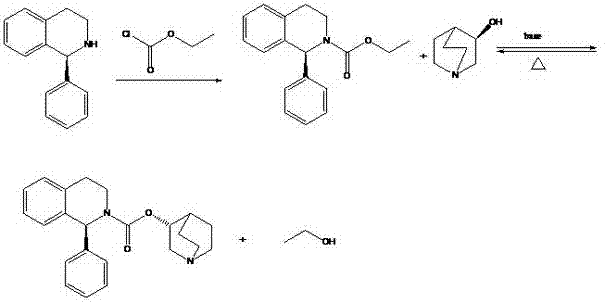
ActiveCN102875544AImprove conversion rateReverse reaction facilitationOrganic chemistrySolifenacin SuccinateCondensation process
The invention relates to a preparation technology of solifenacin succinate. According to the preparation technology, (S)-1-phenyl-1, 2, 3, 4-tetrahydroisoquinoline carbamyl chloride (II) and (R)-3-quinuclidine alcohol metal salt (III) are reacted to generate solifenacin alkali, and then the solifenacin alkali is prepared into solifenacin succinate. With the adoption of the preparation technology provided by the invention, nucleophilic species which lead to a reverse reaction can be avoided during condensation process, namely, the reverse reaction cannot be generated in the preparation technology, therefore, a transformation rate of the solifenacin succinate is improved, a reaction is greatly promoted, a reaction process is quickly carried out under a mild condition, and the preparation technology is suitable for large-scale production.
Owner:CHENGDU SINO STRONG PHARMA
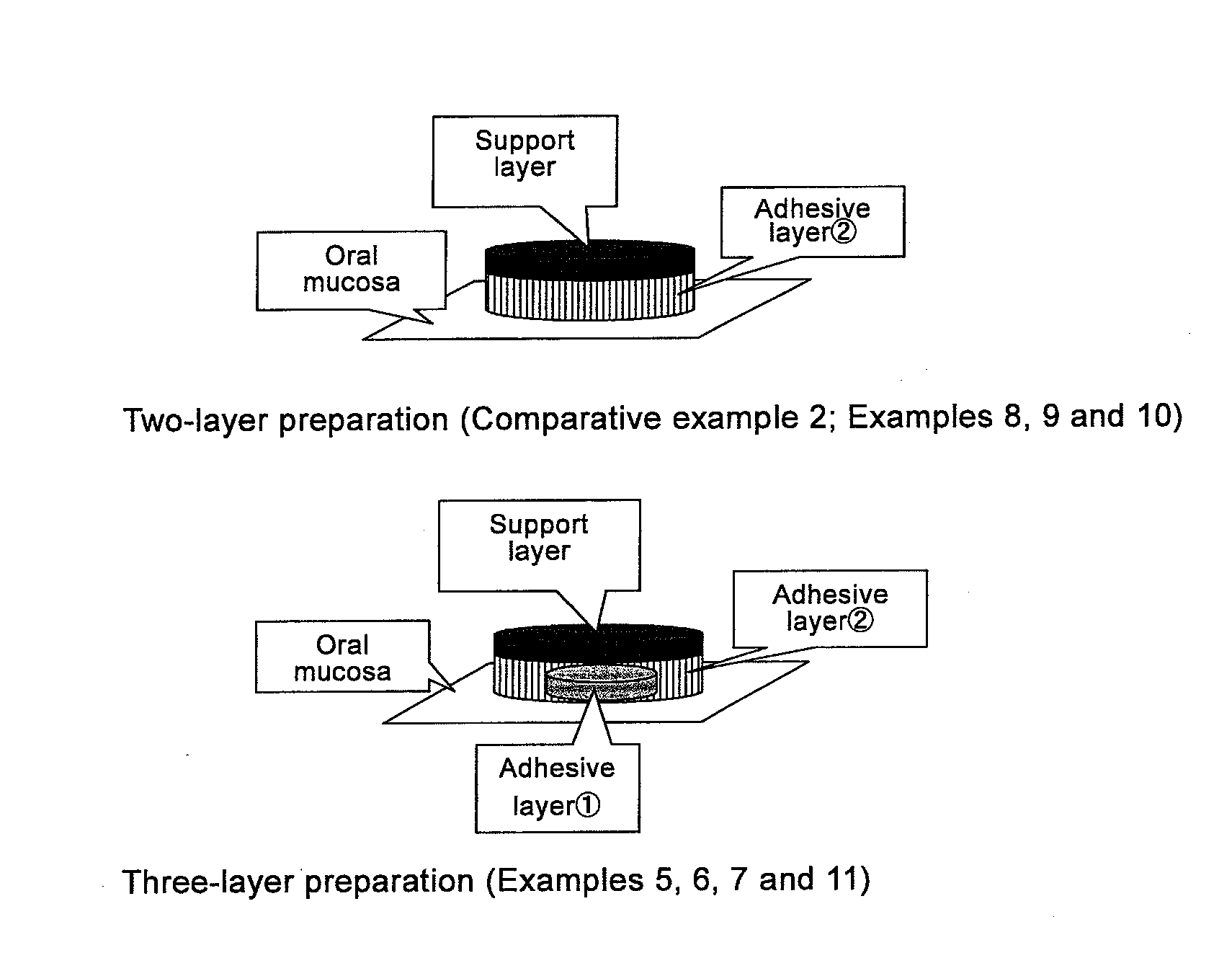
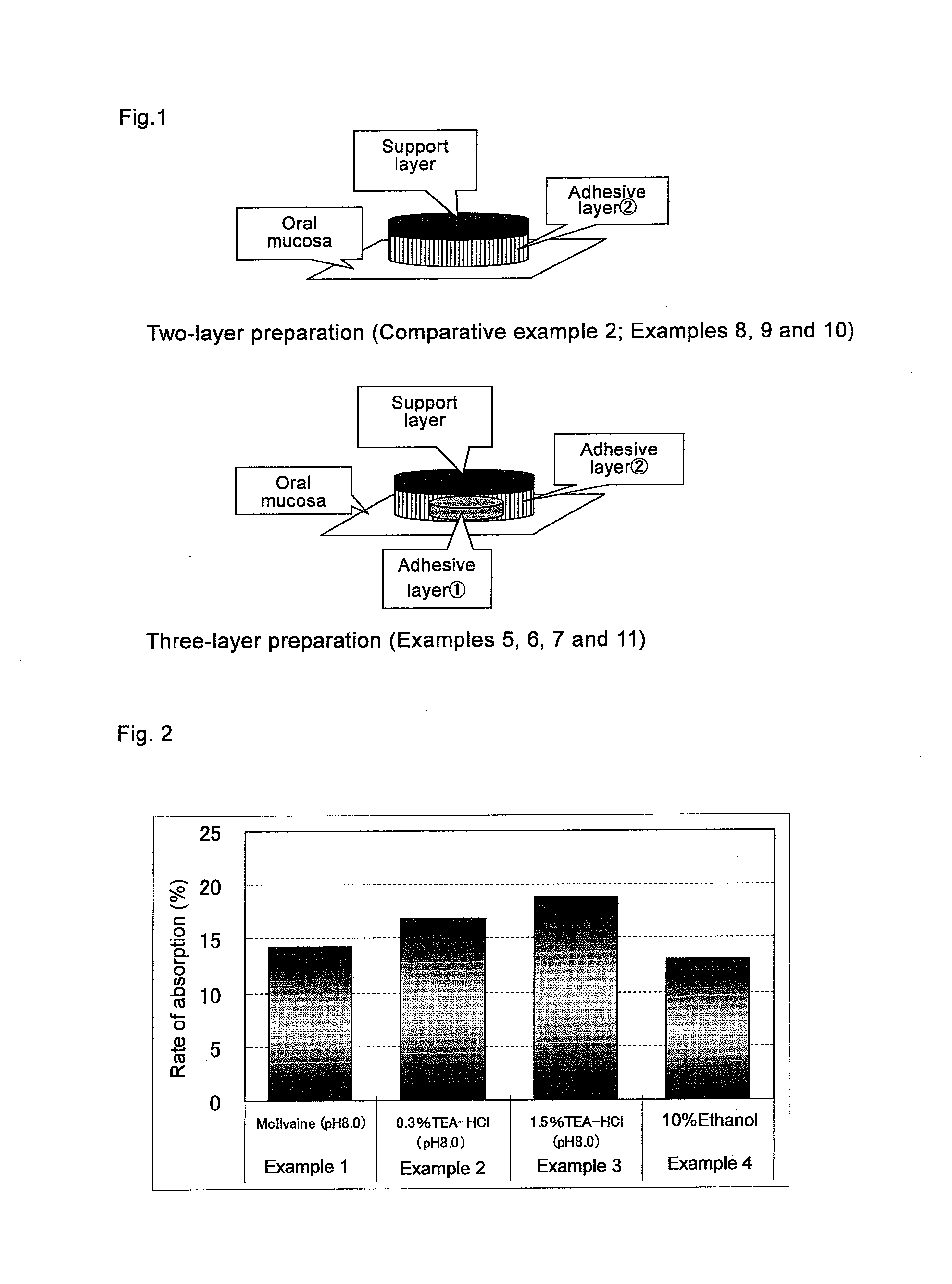
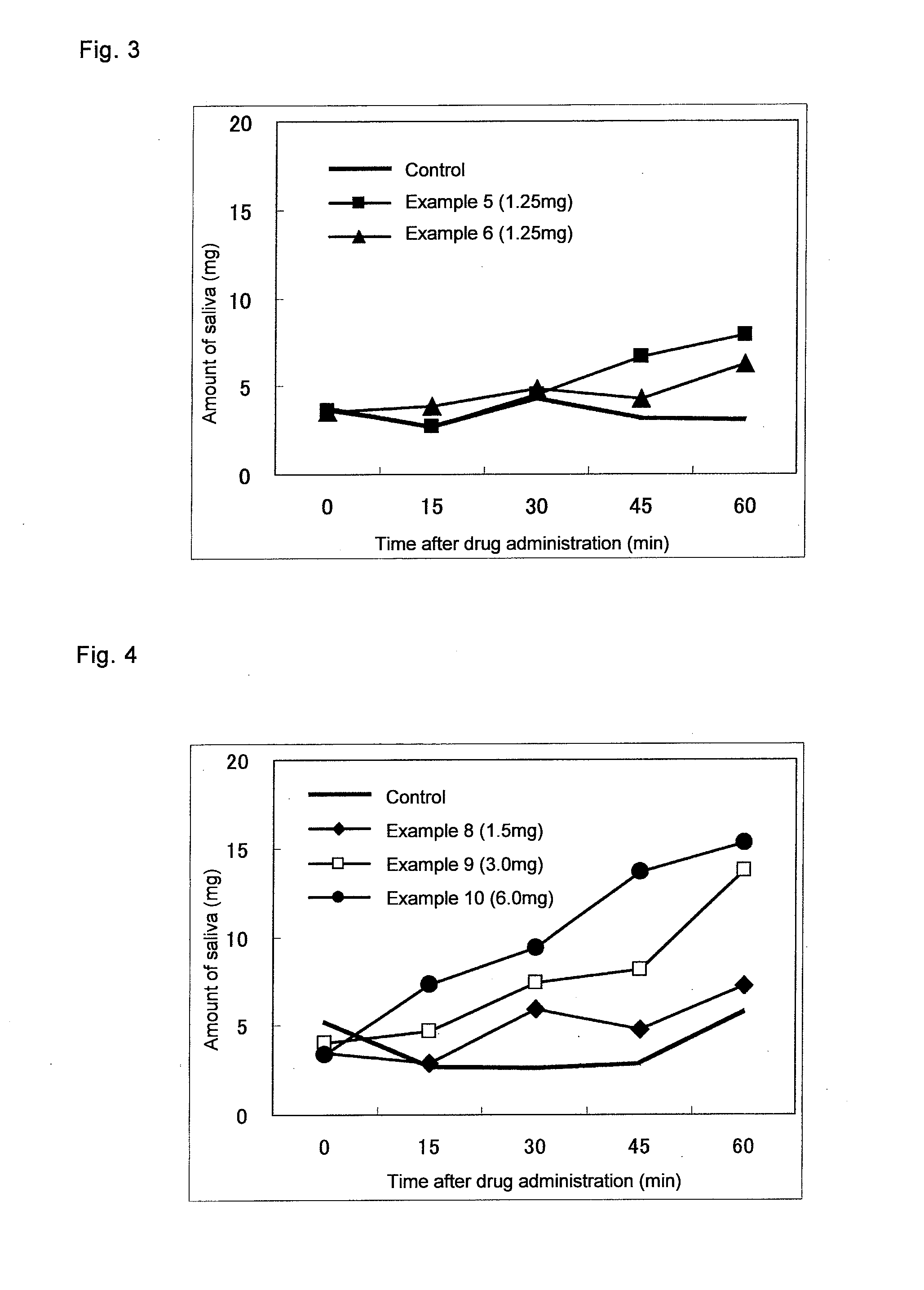
Agent For Oral Mucosal Administration
InactiveUS20110150974A1Superior saliva secretion promotingReduce allocationBiocideOrganic chemistryArylHydrogen atom
A medicament used for prophylactic and / or therapeutic treatment of xerostomia, which is in the form for oral mucosal administration comprising a spirooxathiolane quinuclidine derivative represented by the following general formula (I) or an acid addition salt thereof:[Formula 1](wherein R1 and R2 may be the same or different, and independently represent a hydrogen atom, an alkyl group, a cyclopentyl group, a cyclohexyl group, a monoaryl- or diaryl-substituted methylol group, or an aryl-substituted alkyl group) as an active ingredient.
Owner:DAIICHI PHARMA CO LTD +1
Methods for treating proteinopathies
InactiveUS20180036295A1Reduce and reverse and prevent protein aggregationAmeliorate memory deficitOrganic active ingredientsNervous disorderDementia with Lewy bodiesDisease
This disclosure relates to a method of treating a proteinopathy in a subject, the method comprising administering to the subject an effective amount of a quinuclidine compound. The disclosure also relates to a method of reducing, reversing or preventing the accumulation of protein aggregates in tissue of a subject diagnosed as having a proteinopathy, or being at risk of developing a proteinopathy, the method comprising administering to the subject an effective amount of a quinuclidine compound. Also disclosed is a pharmaceutical composition comprising a quinuclidine compound for use in said methods. The proteinopathy may be a synucleinopathy or a tauopathy, such as Parkinson's disease, Alzheimer's disease or dementia with Lewy bodies.
Owner:GENZYME CORP
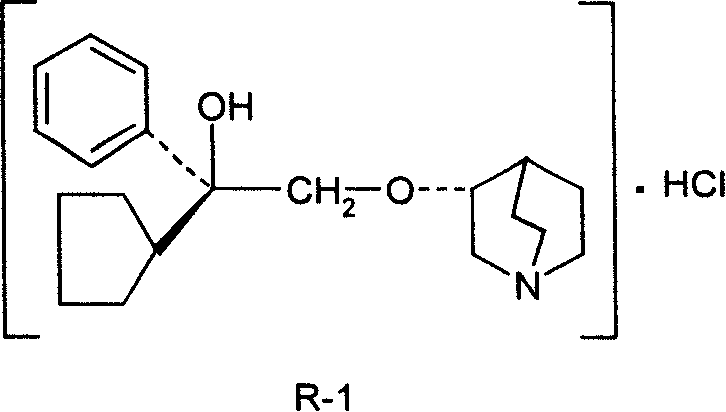
Preparation method of impurity in penehyclidine hydrochloride
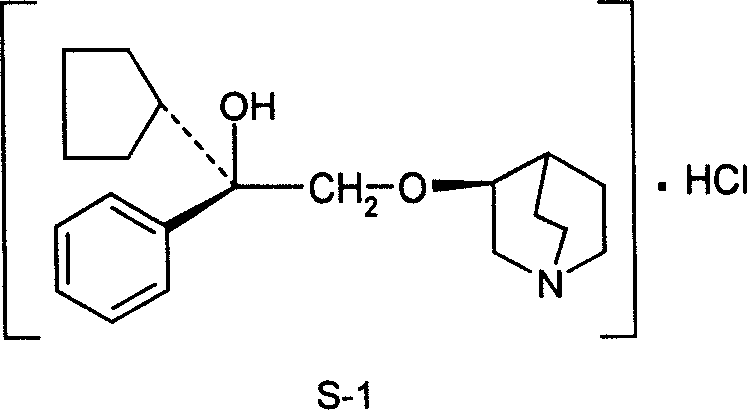
The invention discloses a preparation method of an impurity in penehyclidine hydrochloride. The preparation method comprises the following steps: taking alpha-phenyl-alpha-cyclopentyl-alpha-hydroxyl ethyl p-toluene sulfonate and 3-quinuclidinol as the raw materials, carrying out reactions for a while under an alkaline condition, performing a post treatment to obtain 3-[2-cyclopentyl-2-phenyl-2-(2-cyclopentyl-2-hydroxyl-2-phenyl-ethyoxyl)ethyoxyl] quinuclidine hydrochloride free alkali, carrying out salt forming reactions between the free alkali and hydrogen chloride, and performing refinement to obtain hydrochloride of the free alkali. The preparation method can prominently increase the impurity content during the preparation process, the operation difficulty is reduced, moreover, the preparation method is suitable for massive production and is capable of obtaining a qualified high purity impurity; and the impurity purity measured by HPLC is 100%.
Owner:海南欣莱医药科技股份有限公司
Salts of quinuclidine derivative
There is provided an acid addition salt of (−)-(3R)-quinuclidin-3-yl(1R)-1-phenyl-1,2,3,4-tetrahydroisoquinoline-2-carboxylate with an acid selected from the group consisting of (−)-(2S,3S)-tartaric acid, (+)-(2S,3S)-di-O-benzoyltartaric acid, (+)-(2S,3S)-di-O-(4-methylbenzoyl)tartaric acid, (−)-L-phenylalanine, benzenesulfonic acid, cyclohexanesulfamic acid, hydrobromic acid, naphthalene-2-sulfonic acid, sebacic acid, (+)-camphor-10-sulfonic acid, p-toluenesulfonic acid, ethanesulfonic acid, methanesulfonic acid and methyl phosphate, which has little hygroscopicity that affects the use as a drug or its drug substance, and is very useful as a drug or its drug substance.
Owner:ASTELLAS PHARMA INC
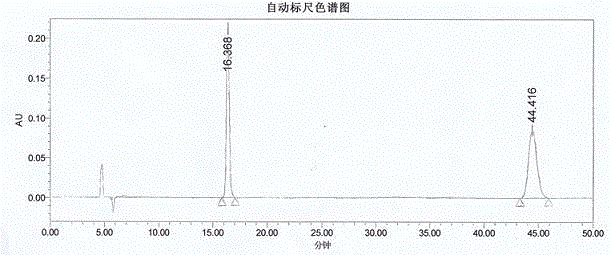
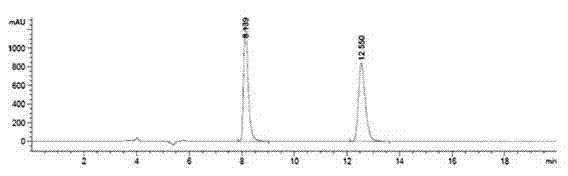
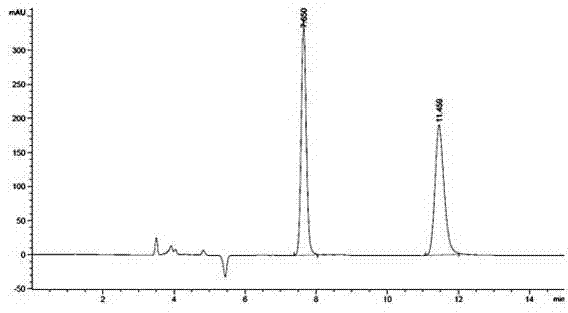
Method for resolving R/S-3-quinuclidinol by adopting precolumn derivation high performance liquid chromatography
ActiveCN104502470AHas UV absorptionWide applicabilityComponent separationFluid phasePhysical chemistry
The invention discloses a method for resolving R / S-3-quinuclidinol by adopting a precolumn derivation high performance liquid chromatography. The method comprises the following steps of firstly, carrying out precolumn derivation esterification reaction on R / S-3-quinuclidinol to obtain R / S-3-quinuclidinol ester; then, preparing a 1.0mg / mL sample solution from R / S-3-quinuclidinol ester by using a high performance liquid chromatograph, wherein an amylose chiral column is used as a chromatographic column, a mixed solution of n-hexane, an alcohol and an alkaline additive is used as a positive mobile phase, and the flow rate, the sample volume, the detection wavelength and the chromatographic column temperature are controlled at 0.6-1mL / min, 5mul, 220-280nm and 20-35 DEG C respectively; and resolving the R / S-3-quinuclidinol ester chirally. The chromatographic peak obtained by adopting the method is good in pattern and symmetry and high in degree of resolving, and the method can be used for detecting and resolving R / S-3-quinuclidinol efficiently, rapidly and sensitively.
Owner:上海柏狮生物科技有限公司
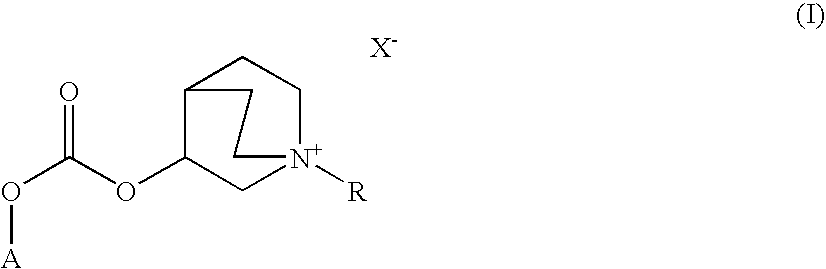
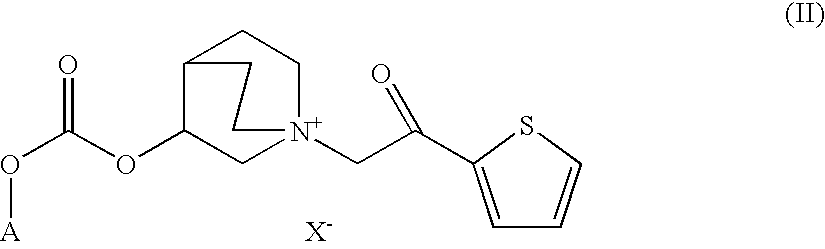
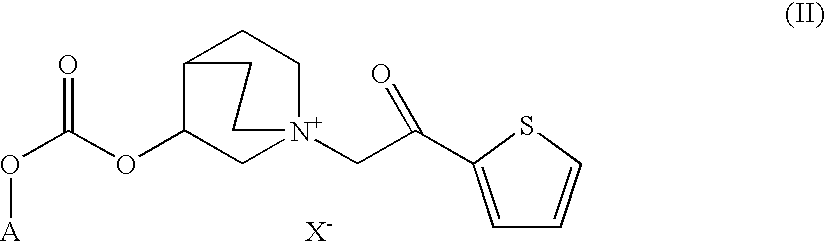
Quinuclidine carbonate salts and medicinal composition thereof
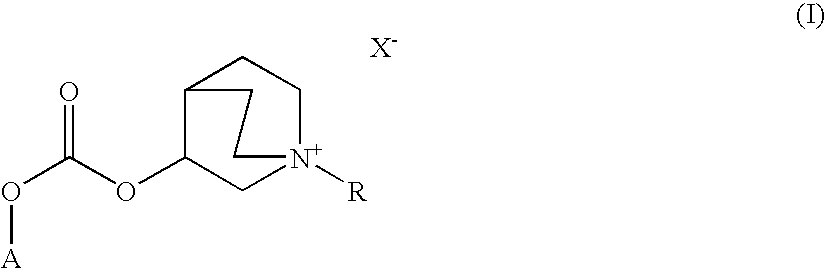
ActiveUS20100035922A1Improve performanceLong duration of actionRespiratorsBiocideDiseasePharmacology
Quinuclidine carbonate derivatives act as muscarinic receptor antagonists and are effective for the prevention and / or treatment of a broncho-obstructive or inflammatory diseases.
Owner:CHIESI FARM SPA
Geminal substituted quinuclidine amide compounds as agonists of alpha-7 nicotonic acetylcholine receptors
InactiveUS20170369486A1Significant positive effectIncrease awarenessOrganic active ingredientsNervous disorderAcetylcholine receptorCognitive defects
The present invention relates to novel geminal substituted quinuclidine amide compounds, and pharmaceutical compositions of the same, that are suitable as agonists or partial agonists of α7-nAChR, and methods of preparing these compounds and compositions, and the use of these compounds and compositions in methods of maintaining, treating and / or improving cognitive function. In particular, methods of administering the compound or composition to a patient in need thereof, for example a patient with a cognitive deficiency and / or a desire to enhance cognitive function, that may derive a benefit therefrom.
Owner:AXOVANT SCI GMBH
Novel quinuclidine derivatives and their use
This invention relates to novel quinuclidine derivatives and their use as pharmaceuticals. Due to their pharmacological profile the compounds of the invention may be useful for the treatment of diseases or disorders as diverse as those related to the cholinergic system of the central nervous system (CNS), the peripheral nervous system (PNS), diseases or disorders related to smooth muscle contraction, endocrine diseases or disorders, diseases or disorders related to neuro-degeneration, diseases or disorders related to inflammation, pain, and withdrawal symptoms caused by the termination of abuse of chemical substances.
Owner:NEUROSEARCH AS
Quinuclidine carbonate salts and medicinal composition thereof
ActiveUS8039483B2Improve performanceLong duration of actionBiocideOrganic chemistryPharmaceutical SubstancesMuscarinics
Owner:CHIESI FARM SPA
Application of fluoro-diphenyl sulfimide as nitrogen heterocyclic Diels-Alder reaction catalyst
InactiveCN101797519AHigh yieldReduce pollutionOrganic chemistryOrganic-compounds/hydrides/coordination-complexes catalystsNitrogenSolvent
The invention discloses an application of fluoro-diphenyl sulfimide as a nitrogen heterocyclic Diels-Alder reaction catalyst and has the advantages of high catalytic activity, good three-dimensional selectivity, low cost, easy acquisition, and the like. The invention also provides a method for synthesizing an iso-quinuclidine compound (I) by using the fluoro-diphenyl sulfimide as the nitrogen heterocyclic Diels-Alder reaction catalyst and catalyzing cyclonene, aromatic aldehyde (ii) and aromatic amine (iii) in an inorganic solvent or an organic solvent to carry out a nitrogen heterocyclic Diels-Alder reaction. The invention has the advantages of simple, convenient and rapid operation, high product yield, good three-dimensional selectivity, less environmental pollution, low production cost, and the like, thereby having good prospects.
Owner:SOUTHWEST UNIVERSITY
Nitrogen oxide purification CHA zeolite molecular sieve as well as preparation method and application of catalyst thereof
PendingCN111871454AReasonably acidicGood hydrothermal stabilityInternal combustion piston enginesMolecular sieve catalystsMolecular sievePtru catalyst
The invention discloses a nitrogen oxide purification CHA zeolite molecular sieve as well as a preparation method and application of a catalyst thereof, and belongs to the field of chemical synthesistechnologies and application thereof. The CHA zeolite molecular sieve is synthesized by adopting an N,N,N-trialkyl-quinuclidine-4-quaternary ammonium onium compound as an organic template agent, the molar ratio range of silicon dioxide to aluminum oxide is 6-80, the average grain diameter is less than or equal to 500 nm, the total specific surface area is more than or equal to 400m2 / g, the total pore volume is more than or equal to 0.20 ml / g, the micropore volume is more than or equal to 0.10 ml / g, and the grain diameter in the crystal face (-210) direction of the molecular sieve is 50-160 nm.After hydrothermal treatment of the molecular sieve at 600-800 DEG C, the tetra-coordinated aluminum accounts for more than or equal to 90% of the total aluminum amount, and the hexa-coordinated aluminum accounts for less than or equal to 10% of the total aluminum amount. The molecular sieve provided by the invention has high hydrothermal stability without large crystal grains, shows high nitrogen oxide reduction characteristics after being exposed at high temperature and high humidity, and especially shows a catalyst with high nitrogen oxide reduction characteristics in a temperature range of 200-550 DEG C.
Owner:CHINA CATALYST HLDG CO LTD
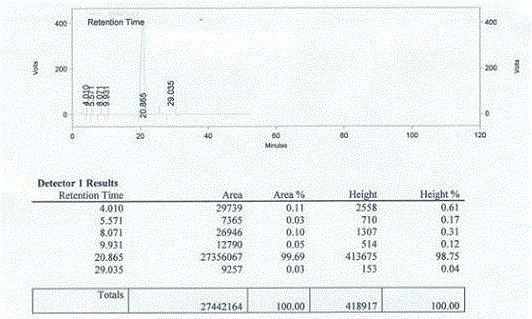
Method of detecting impurities in penehyclidine hydrochloride injection
ActiveCN104237394AImprove controllabilityDirect analysis methodComponent separationVapor phase chromatographyOrganosolv
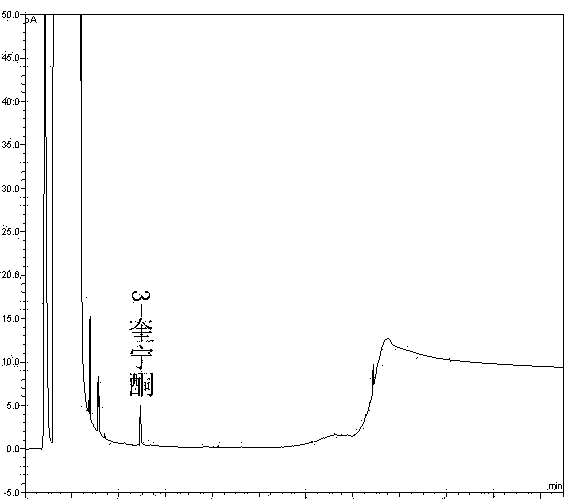
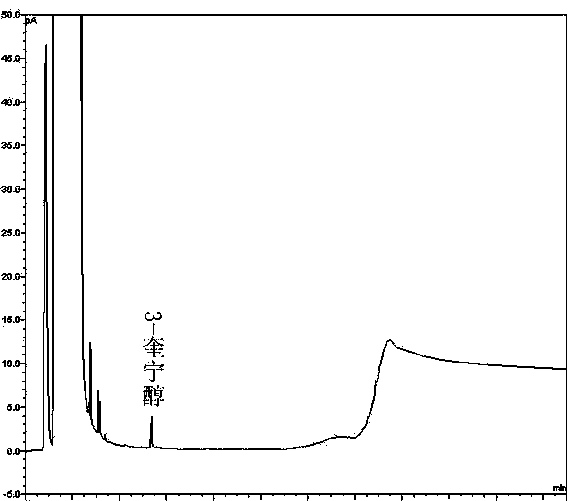
The invention discloses a method of detecting two impurities containing a quinuclidine ring in a penehyclidine hydrochloride injection. The method adopts a gas chromatographic method to detect the contents of the impurities, namely the content of 3-quinuclidone and the content of 3-quinuclidinol. During detection, a sample of the penehyclidine hydrochloride injection is subjected to vacuum rotary evaporation to remove the solvent in the injection, is dissolved with an alkaline organic solvent so as to convert all the hydrochlorides into free alkalis and is dried by blowing nitrogen, and then dimethylformamide is added to prepare a sample solution to be detected; and impurity reference substances are salified by adding an acid firstly, then is processed as the same processing manner for the sample solution to be detected, and is prepared into impurity reference substance solutions. The sample solution to be detected and the impurity reference substance solutions are directly injected respectively, chromatograms are collected, and the contents of the impurities are calculated based on peak areas by an external standard method. The method of detecting the impurities has characteristics of simple and convenient operation, high sensitivity, capability of quantitative measurement, high accuracy and good reproducibility, effectively controls the product quality of the penehyclidine hydrochloride injection, and guarantees safety and effectiveness of clinical medication.
Owner:CHONGQING XIANYANG PHARMA TECH CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com