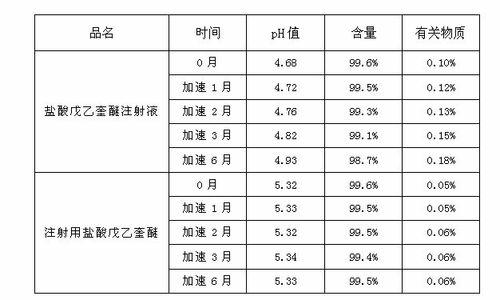
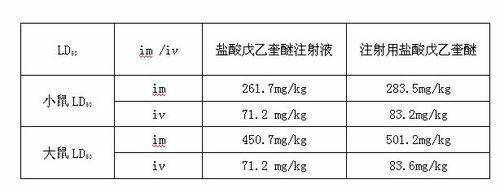
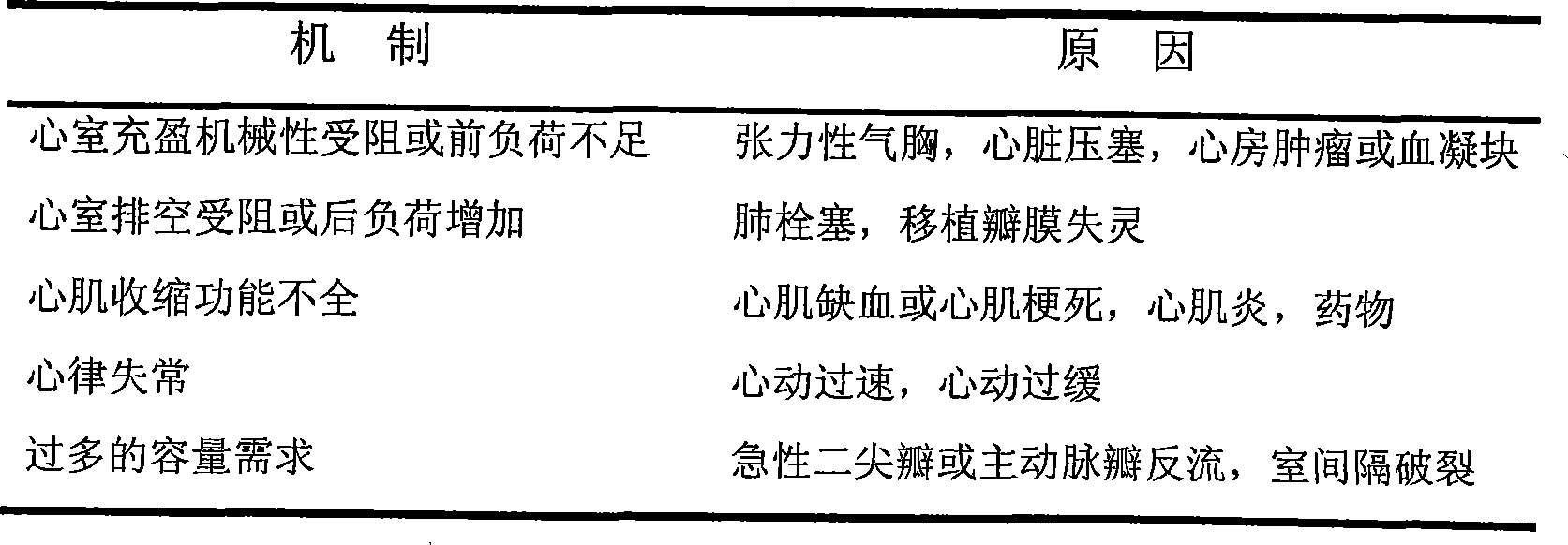
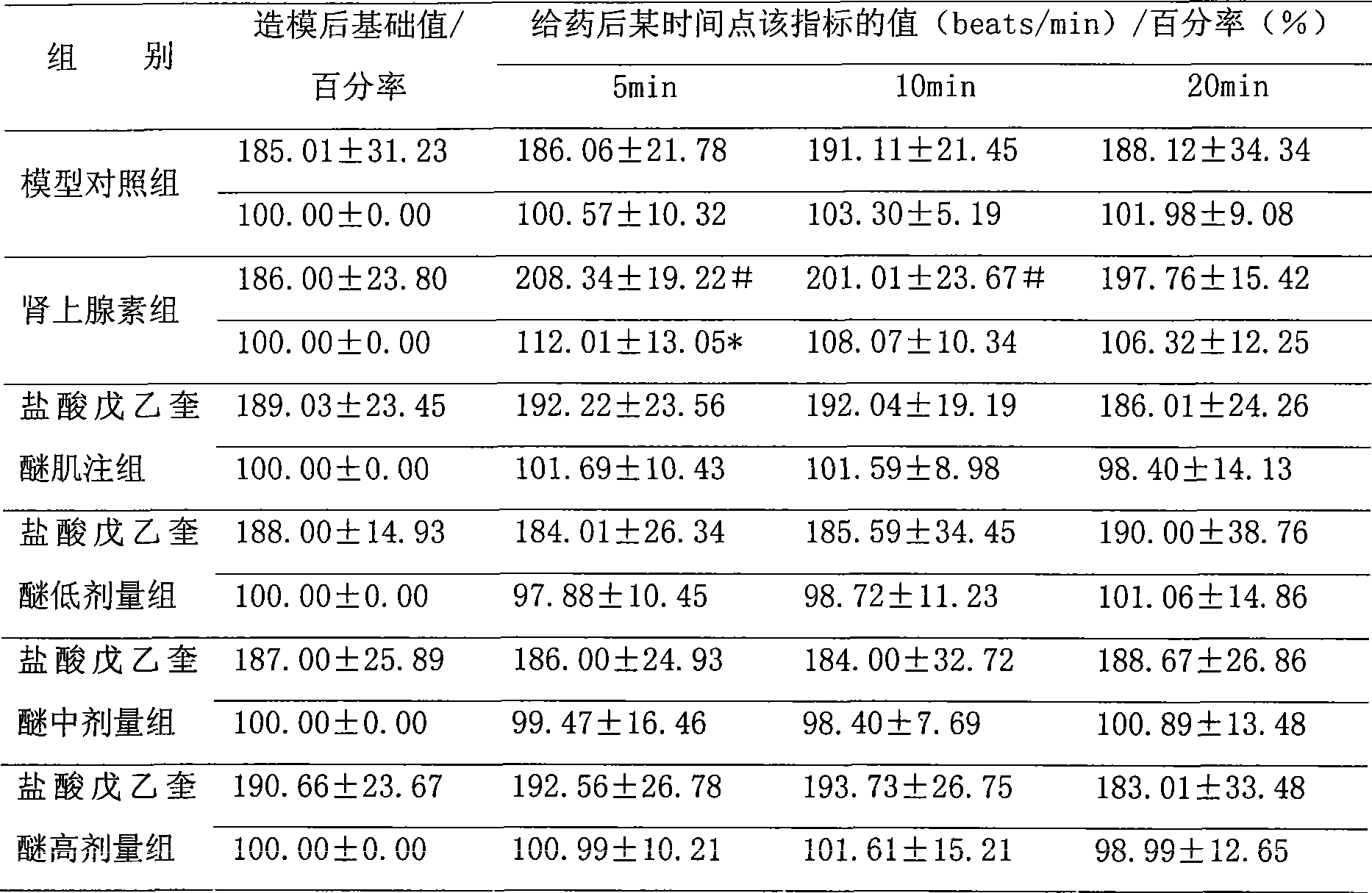
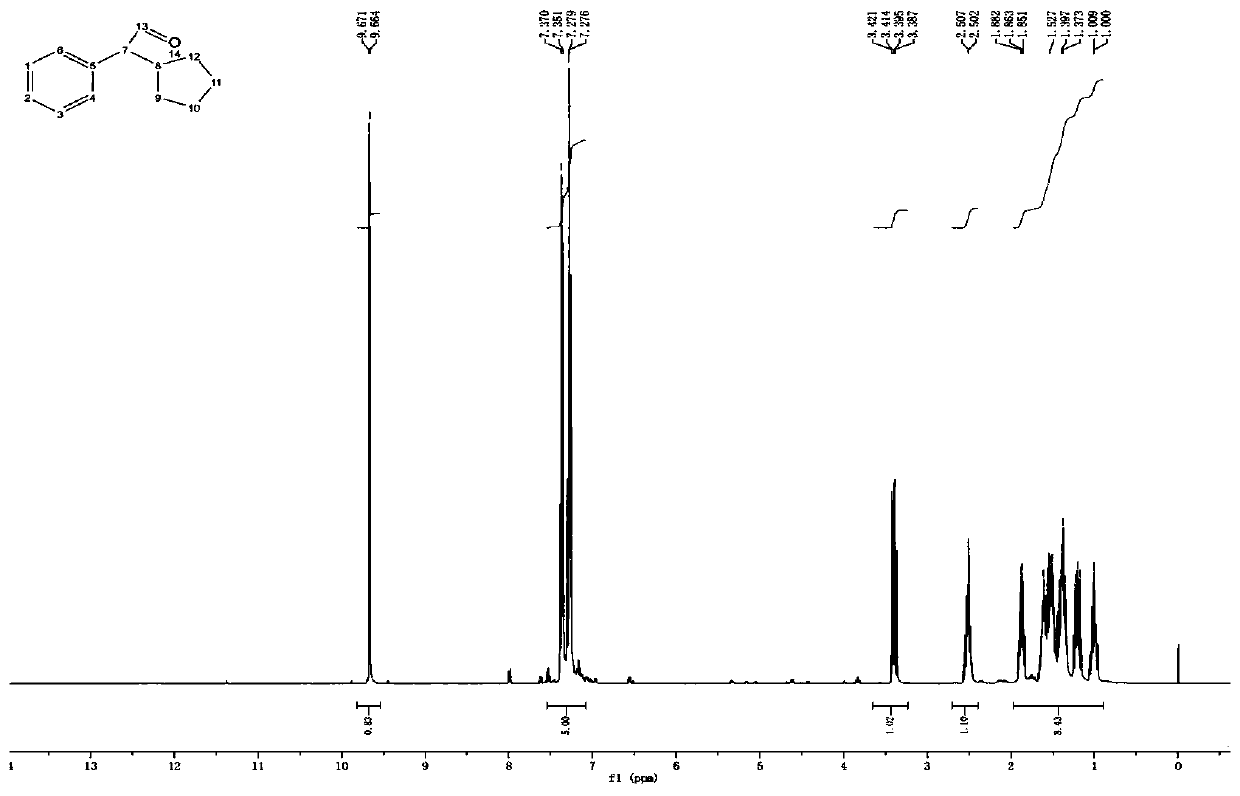
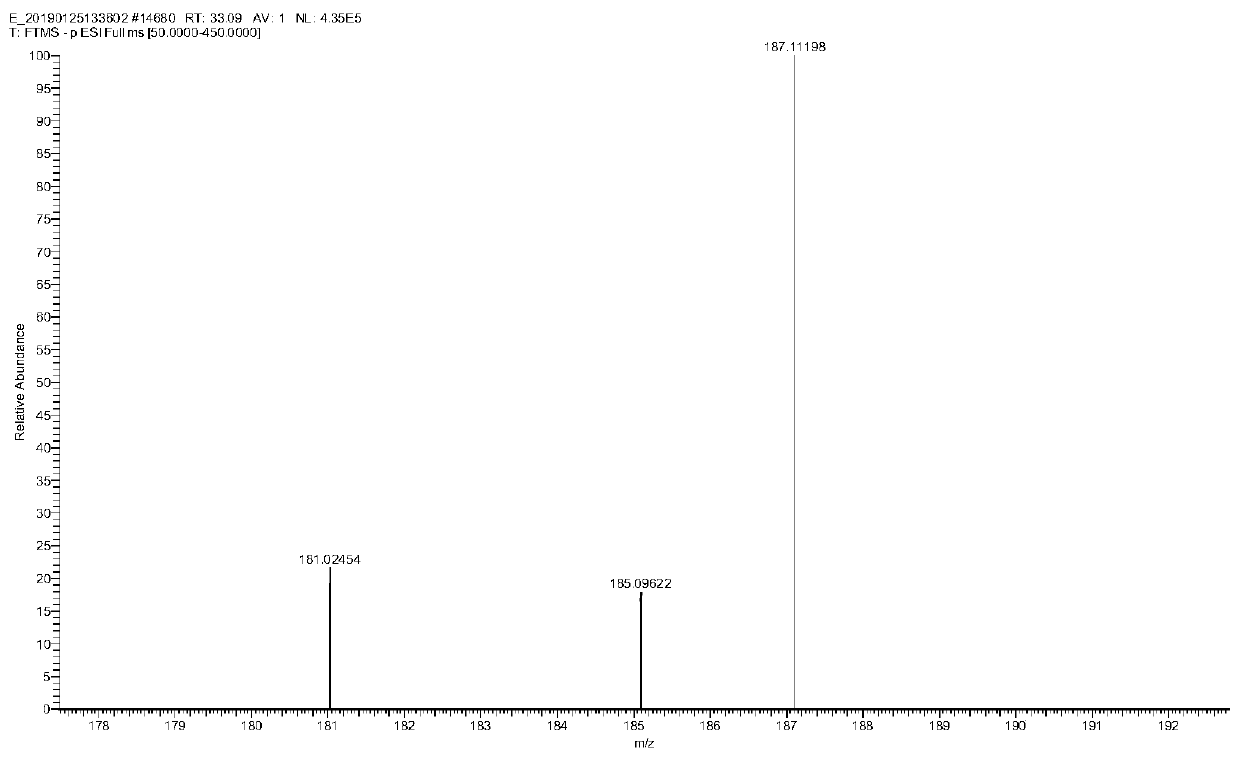
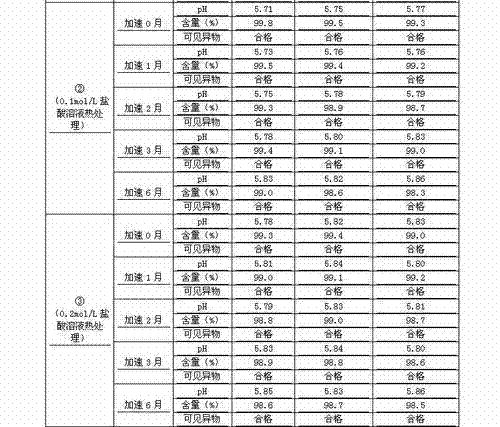
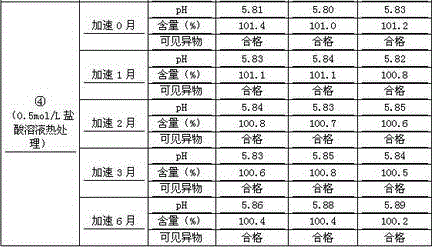
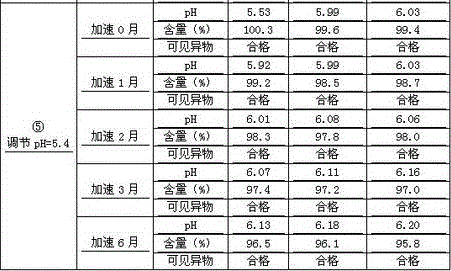
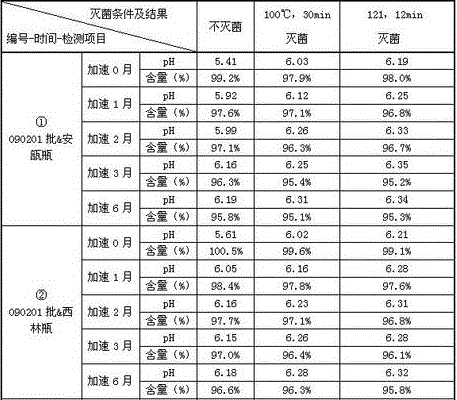
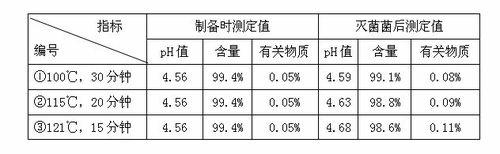
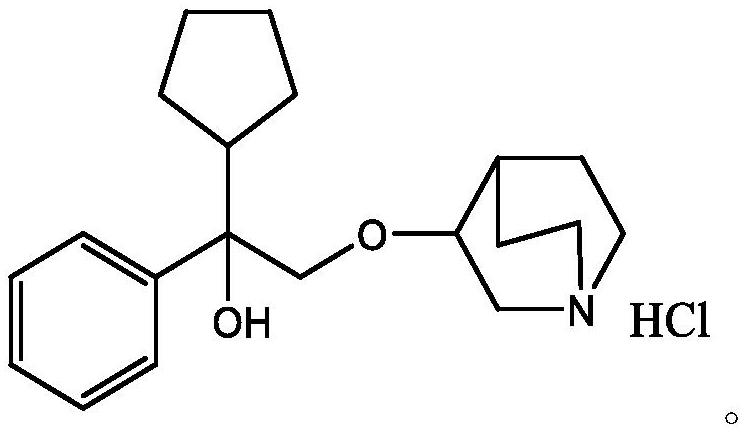
Patents
Literature
59 results about "Penehyclidine hydrochloride" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Application of penehyclidine hydrochloride in preparing medicament for treating haemorrhagic shock
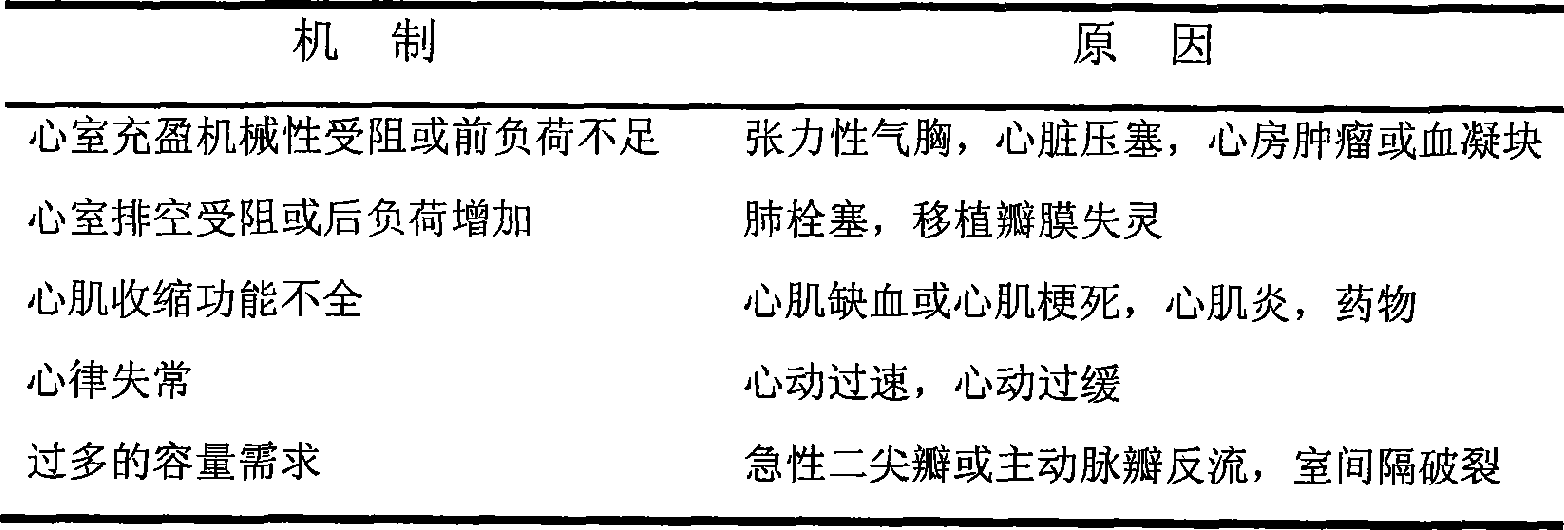
The invention discloses application of penehyclidine hydrochloride in preparation of a medicine for treating a hemorrhagic shock disease. Hemorrhagic shock has a pathological change that the central venous pressure, the peripheral mean arterial pressure and the cardiac output are all obviously reduced and the middle-light microcirculatory disturbance appears, the medicine for treating the hemorrhagic shock disease can be liquid and can also be solid, and the mass percent concentration of the penehyclidine hydrochloride in the medicine is between 0.01 and 2 percent. The application effect is proved through the influence of a penehyclidine hydrochloride water solution on a canine hemorrhagic shock model.
Owner:CHENGDU LIST PHARMA
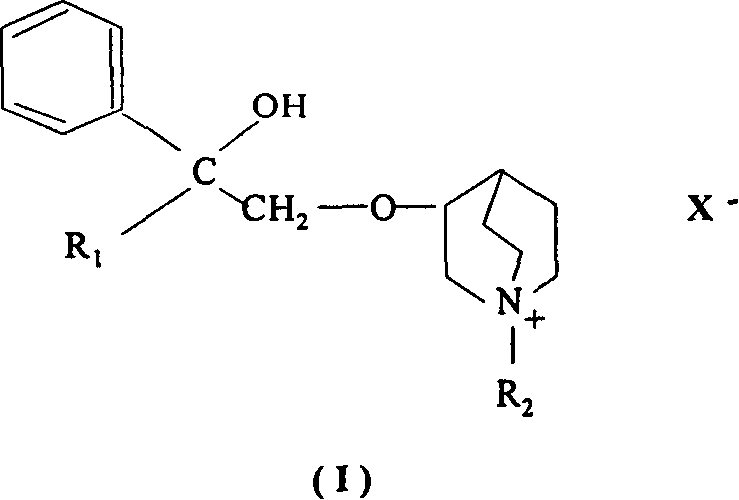
Application of penehyliclidine hydrochloride in preparing medicine
InactiveCN1739511AEffective for abdominal painEffective for biliary colicOrganic active ingredientsAntiinfectivesCurative effectPharmacology
The present invention relates to the application of penehyclidine hydrochloride in preparing medicine, and is especially the application of penehyclidine hydrochloride in preparing medicine for resisting infectious shock. Animal experiment and clinical observation prove the curative effect of penehyclidine hydrochloride in resisting infectious shock and improving micro circulation. The present invention expands the medicinal use of penehyclidine hydrochloride.
Owner:CHENGDU LIST PHARMA
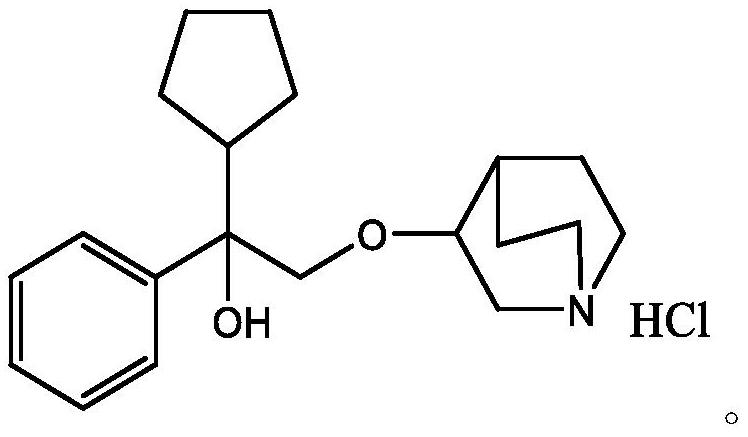
Application of amyl ethyl quin ether hydrochloride
InactiveCN1475214AEffective for abdominal painEffective for biliary colicOrganic active ingredientsSenses disorderDiseaseCurative effect
A new application of pentaethylquinether hydrochloride in treating gastroenteropathy, ophthalmopathy, disease in respiratory tract, disease in central nerve system, etc is disclosed, which features high curative effect.
Owner:JINZHOU AHON PHARM CO LTD
Compound and pharmaceutical composition for treating nasal oversecreation and chronic obstructive pulmonary disease
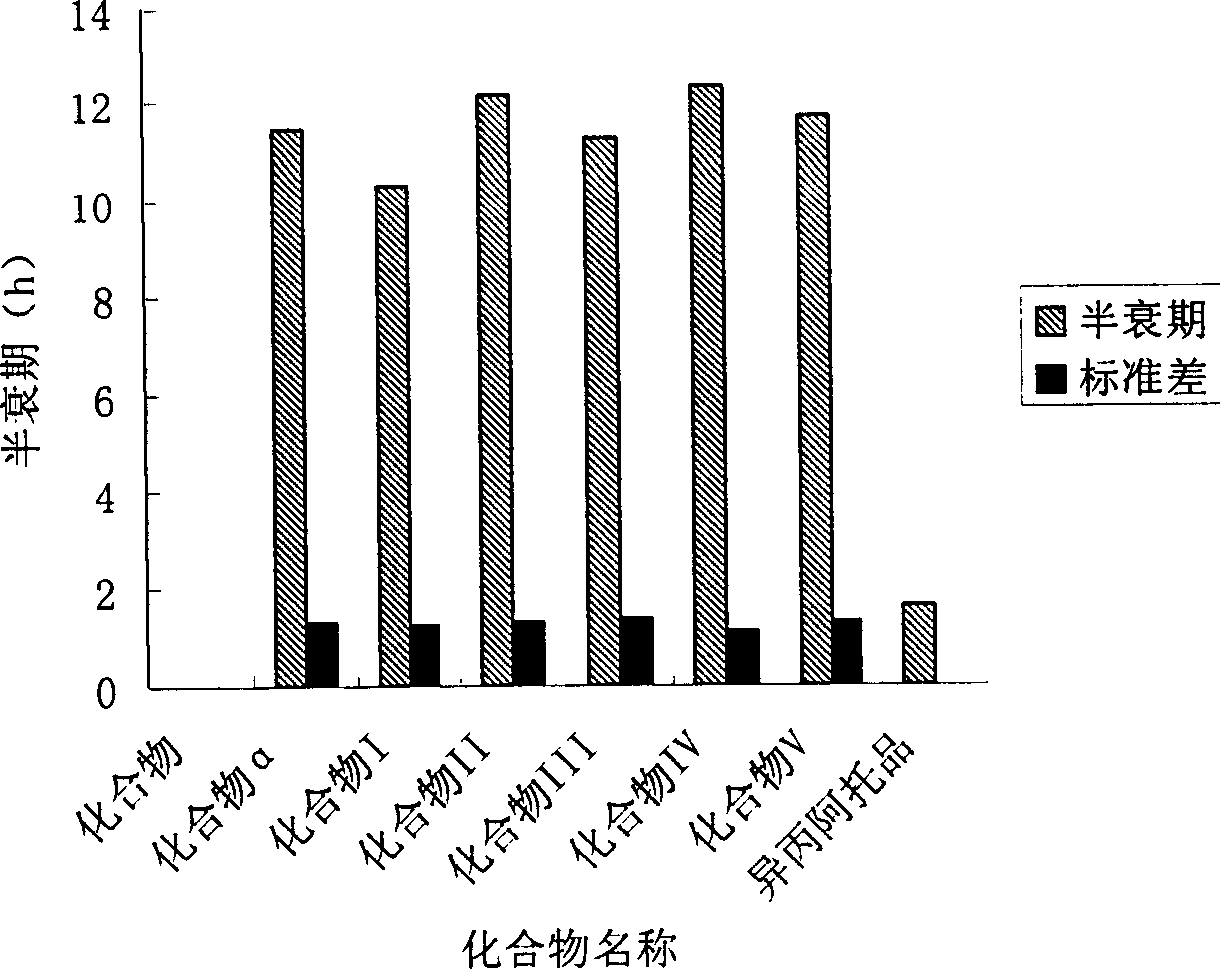
ActiveCN1769286AExtended half-lifeReduce the number of medicationsPowder deliveryOrganic active ingredientsNasal cavityObstructive Pulmonary Diseases
The invention discloses the drug of treating the nasal over-secretion and chronic obstructive emphysema, at the mean time, provides the drug compositions of treating the nasal over-secretion and chronic obstructive emphysema, which are aerosol, powder cloud agent, naristillae and spray. In the invention, the compositions, which are introduced quaternary ammonium group on the basis of penehyclidine hydrochloride, can't permeate membrane and enter into the circulatory system and central, so it can be used in treating the chronic obstructive emphysema. Besides, due to the low absorptivity in the mucous membrane position, the said new compositions are extended the resistance time at local, so it can be used in treating the chronic obstructive emphysema and coryza.
Owner:YINGU PHARMA
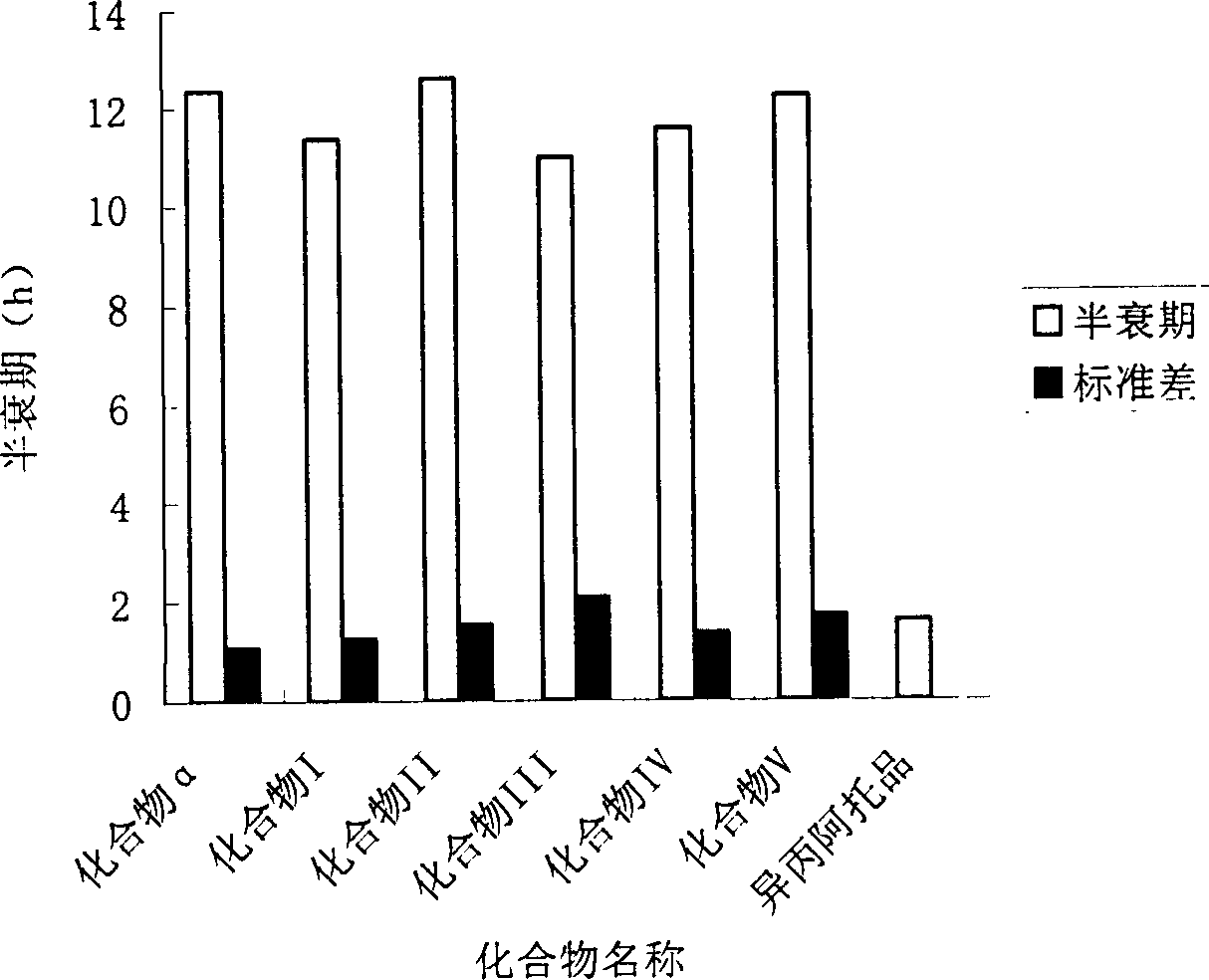
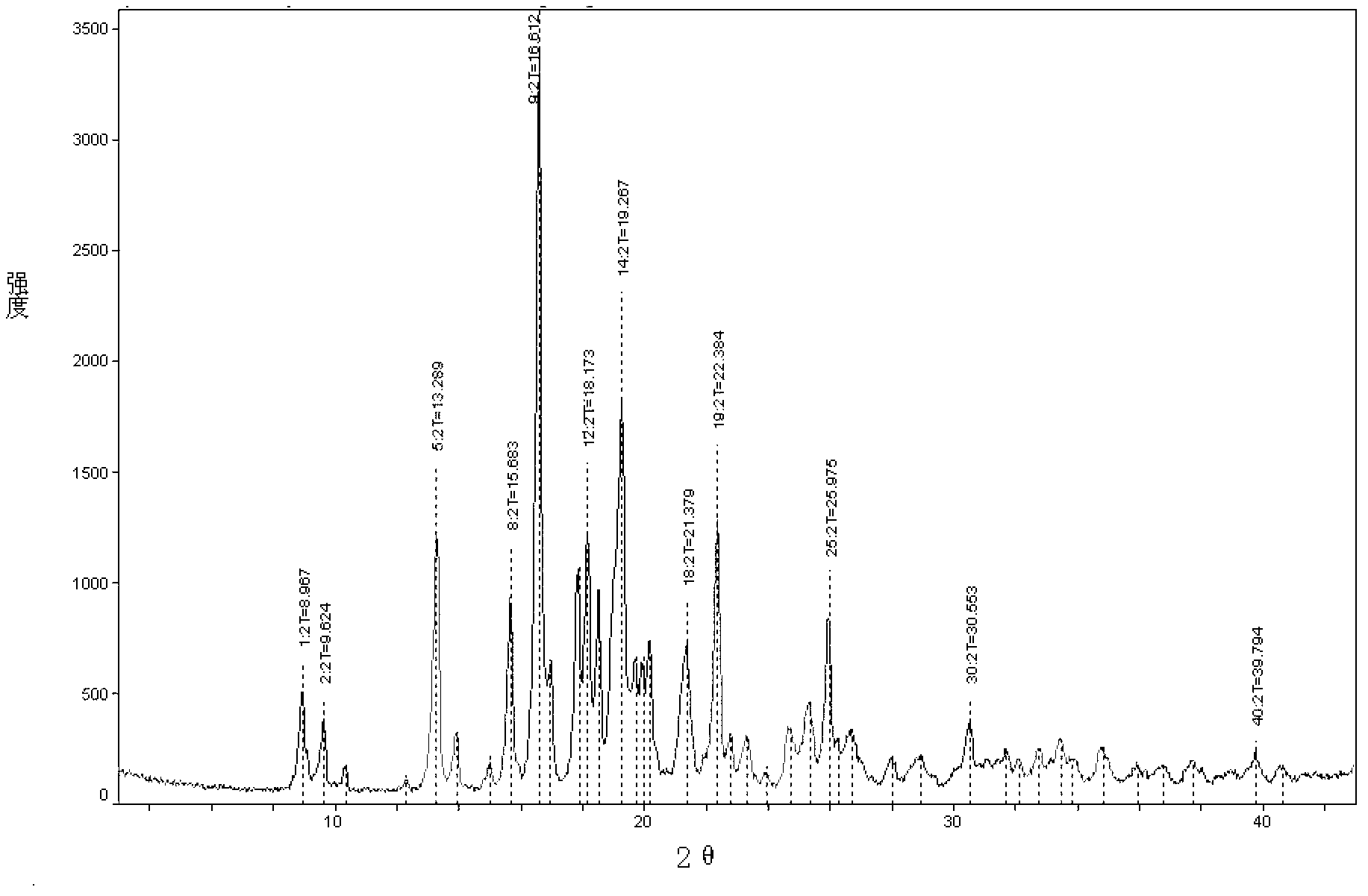
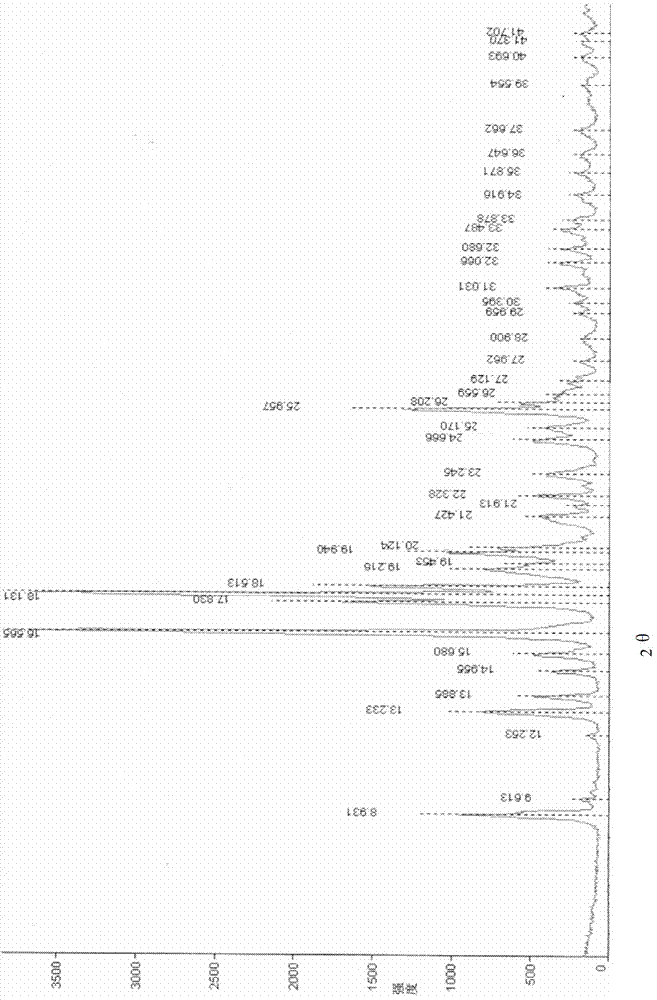
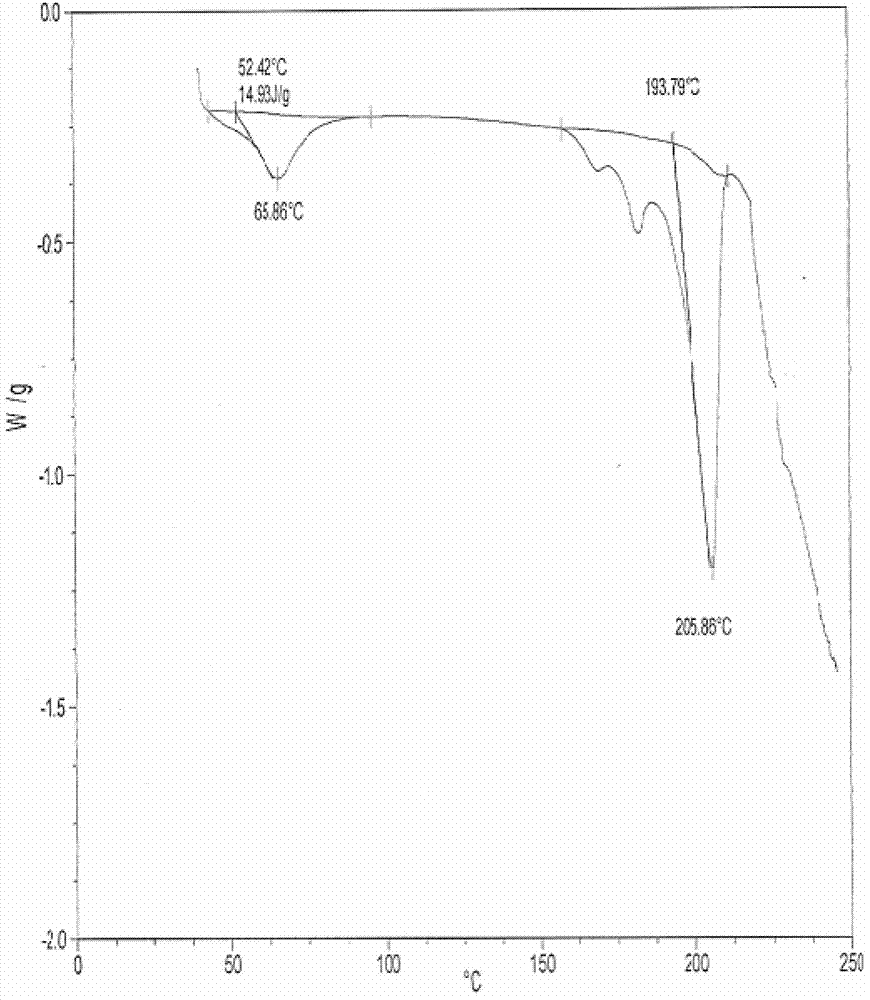
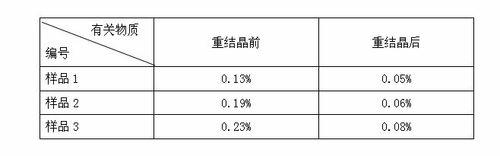
New crystal form of penehyclidine hydrochloride and preparation method of new crystal form
ActiveCN102702187AImprove stabilityEasy to solveOrganic active ingredientsOrganic chemistryClinical efficacyX-ray
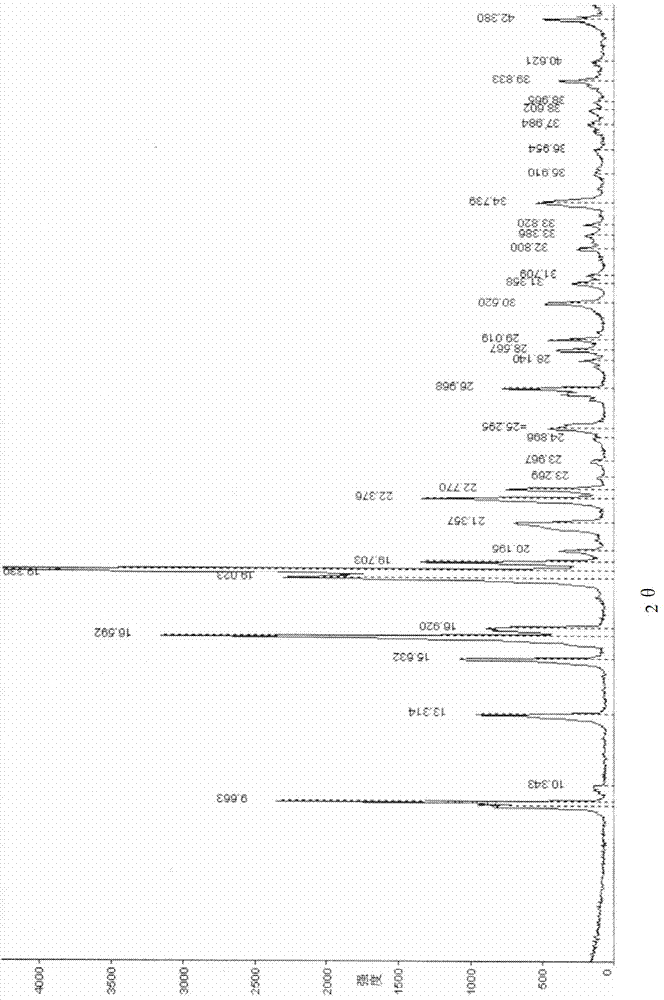
The invention provides a crystal form of penehyclidine hydrochloride. Absorption peaks with diffraction angles (2 theta) at 8.96+ / -0.2, 13.28+ / -0.2, 15.68+ / -0.2, 16.61+ / -0.2, 18.17+ / -0.2, 19.27+ / -0.2, 21.379+ / -0.2, 22.38+ / -0.2 and 25.97+ / -0.2 are available in a powder X-ray diffraction diagram of the crystal form. The invention also provides a preparation method of the crystal form and a medicinal composition based on the crystal form. The crystal form of the penehyclidine hydrochloride has good stability, the increase in medicinal impurities can be effectively avoided without special package and storage conditions, so that the high cost brought by special storage conditions is reduced, long-term research of a non-injection new dosage form of the penehyclidine hydrochloride is facilitated, and effective guarantee is brought to the safety and the clinical therapeutic effect of a final product.
Owner:CHENGDU ZIHAO PHARMA
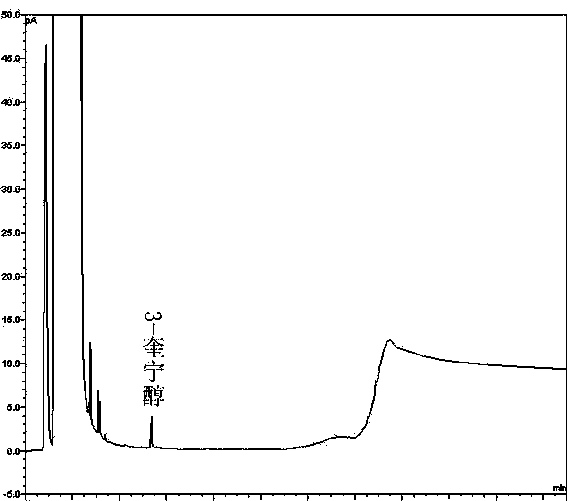
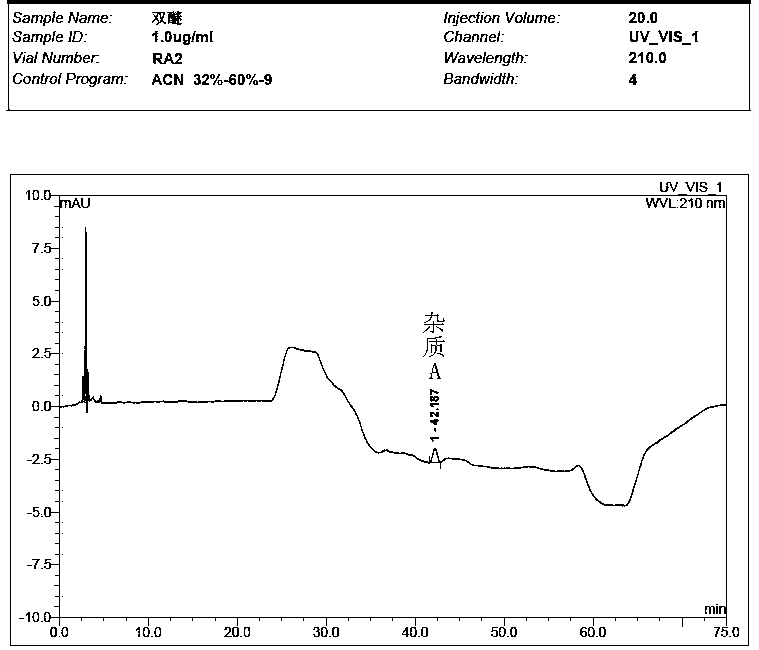
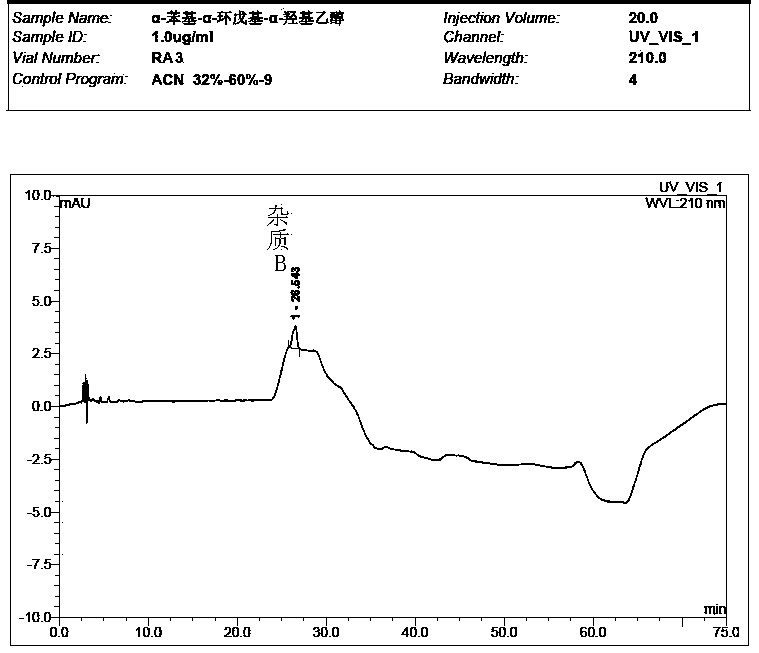
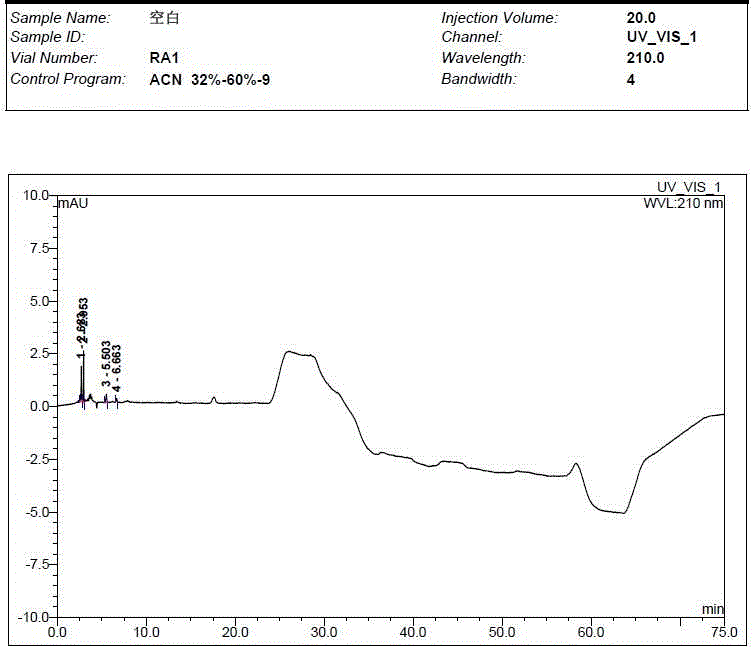
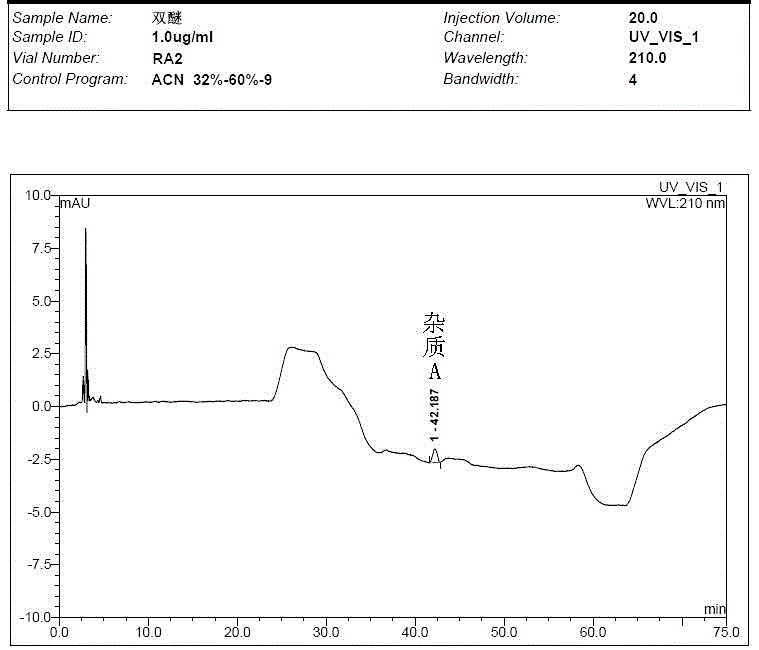
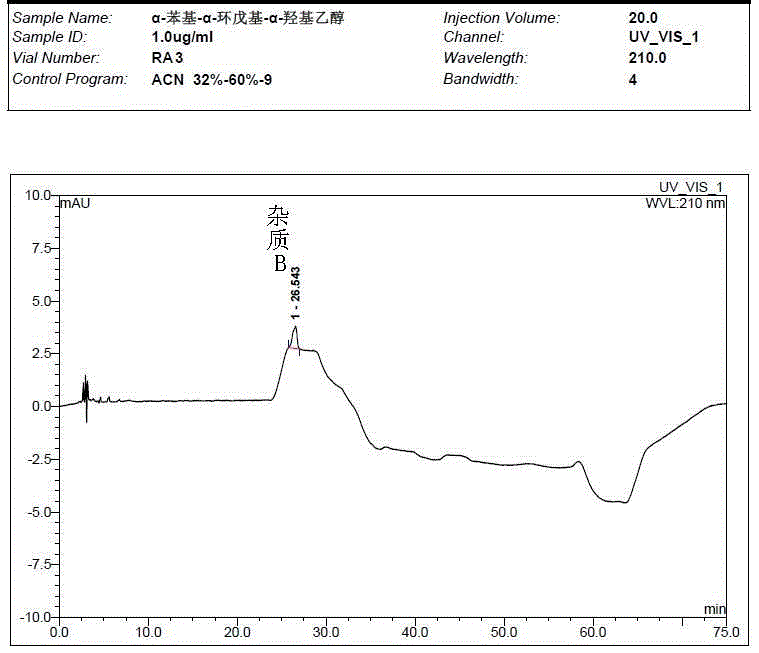
Preparation method of impurity in penehyclidine hydrochloride
The invention discloses a preparation method of an impurity in penehyclidine hydrochloride. The preparation method comprises the following steps: taking alpha-phenyl-alpha-cyclopentyl-alpha-hydroxyl ethyl p-toluene sulfonate and 3-quinuclidinol as the raw materials, carrying out reactions for a while under an alkaline condition, performing a post treatment to obtain 3-[2-cyclopentyl-2-phenyl-2-(2-cyclopentyl-2-hydroxyl-2-phenyl-ethyoxyl)ethyoxyl] quinuclidine hydrochloride free alkali, carrying out salt forming reactions between the free alkali and hydrogen chloride, and performing refinement to obtain hydrochloride of the free alkali. The preparation method can prominently increase the impurity content during the preparation process, the operation difficulty is reduced, moreover, the preparation method is suitable for massive production and is capable of obtaining a qualified high purity impurity; and the impurity purity measured by HPLC is 100%.
Owner:海南欣莱医药科技股份有限公司
Application of penehyclidine hydrochloride in preparing medicine
InactiveCN1739518AEffective for abdominal painEffective for biliary colicOrganic active ingredientsAerosol deliveryPharmacologyGland secretion
he present invention relates to the application of penehyclidine hydrochloride in preparing medicine, and is especially the application of penehyclidine hydrochloride in preparing pre-narcosis medicine. Animal experiment and clinical observation prove the obvious gland secretion inhibiting effect of penehyclidine hydrochloride as pre-narcosis medicine. The present invention expands the medicinal use of penehyclidine hydrochloride.
Owner:CHENGDU LIST PHARMA
Process for preparing penehyclidine hydrochloride injection
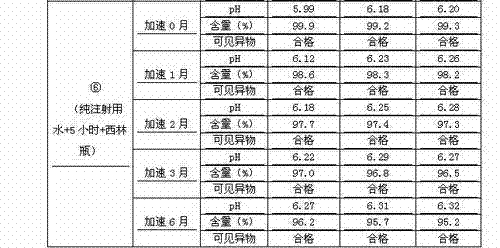
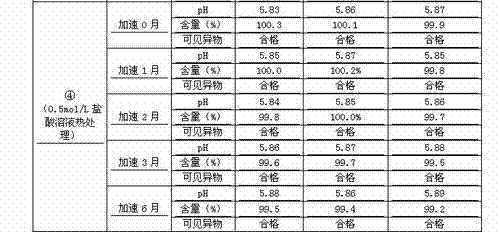
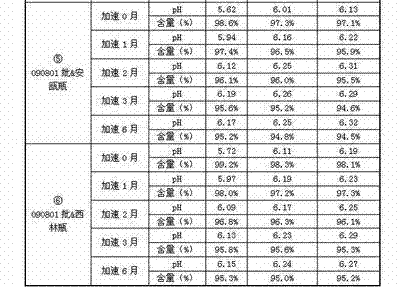
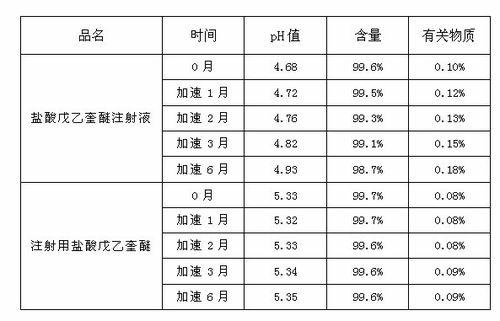
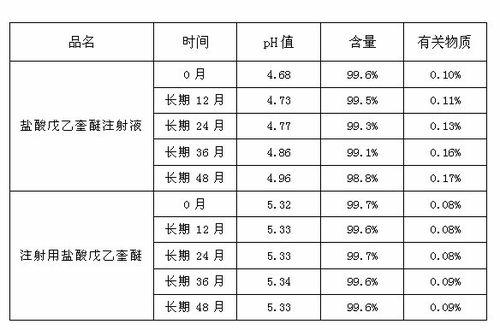
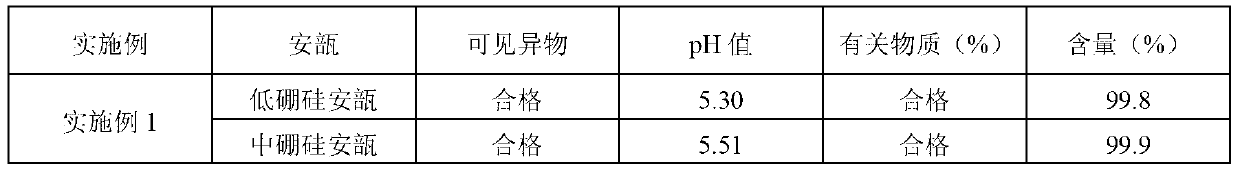
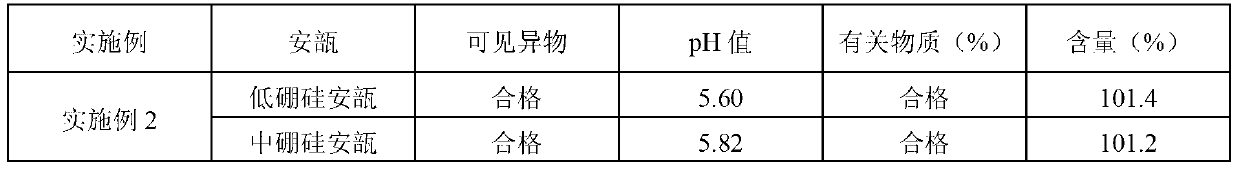
ActiveCN102525910AGuarantee product qualityStable pHOrganic active ingredientsDigestive systemPasteurizationDrug product
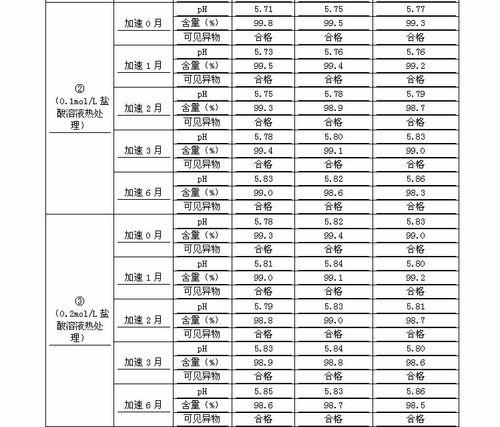
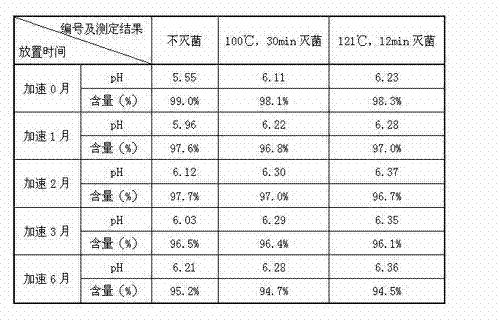
The invention discloses a process for preparing a penehyclidine hydrochloride injection and belongs to the field of medicines. The specific preparation process of the injection is as follows: (1) treating inner packaging materials with acid in advance, then washing the inner packaging materials with purified water and water for injection and then pasteurizing and drying the inner packaging materials for standby application; (2) weighing penehyclidine hydrochloride based on the prescription dose, adding a defined amount of water for injection to dissolve penehyclidine hydrochloride, then adding water for injection to reach the total amount and stirring the liquid medicine uniformly; and (3) measuring the pH value and the content, filtering the liquid medicine after the pH value and the content are qualified, filling and sealing the filtrate in the inner packaging materials for standby application and carrying out pasteurization, thus obtaining the penehyclidine hydrochloride injection.The process has the following beneficial effects: not only can the pH value and content of the penehyclidine hydrochloride injection product obtained by the process be more stable, but also the product quality is more easily ensured; and meanwhile, the final product is proved to be safe and effective by animal long-term toxicity tests and pharmacodynamics tests.
Owner:JINZHOU AHON PHARM CO LTD
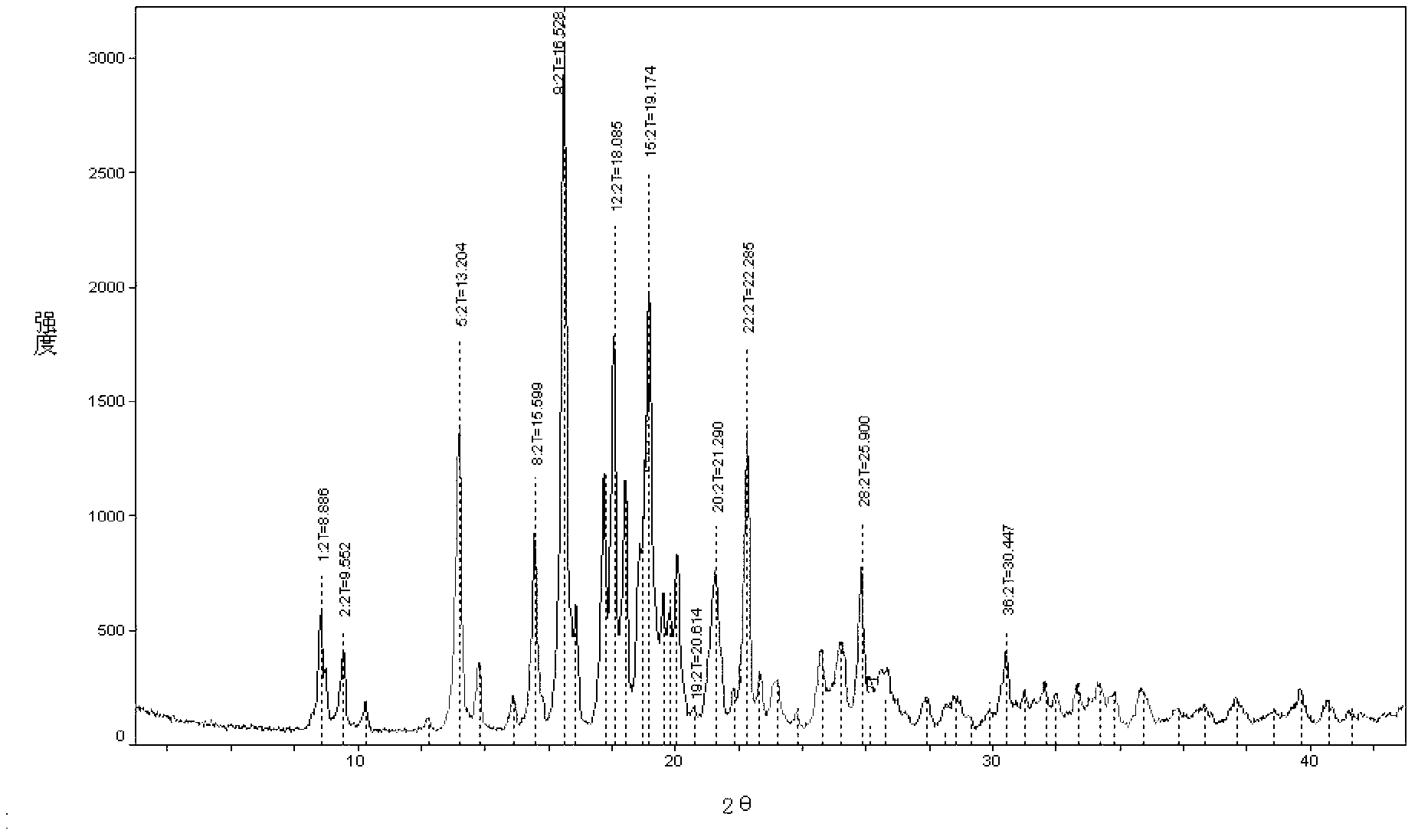
Crystal form of penehyclidine hydrochloride racemic mixture I and preparation method thereof
ActiveCN103483333AReduce humidityPrevent deliquescenceOrganic active ingredientsOrganic chemistryX-rayRacemic mixture
The invention provides a crystal form of a penehyclidine hydrochloride racemic mixture I. In a powder X-ray diffraction pattern of the crystal form, characteristic peaks are achieved when a diffraction angle 2 theta is within the ranges of 9.46-9.86 degrees, 16.39-16.79 degrees, 18.82-19.22 degrees and 19.13-19.53 degrees. The invention further provides a preparation method for the crystal form. According to the invention, the prepared crystal form of the penehyclidine hydrochloride racemic mixture I can obviously reduce the hygroscopicity of the compound, avoids deliquescence, deformation, mildewing or degradation of the compound, which are caused by the fact that water is absorbed by the compound, provides convenience for storage and transportation of the compound, and provides a basis for improvement on the drug stability.
Owner:CHENGDU ZIHAO PHARMA
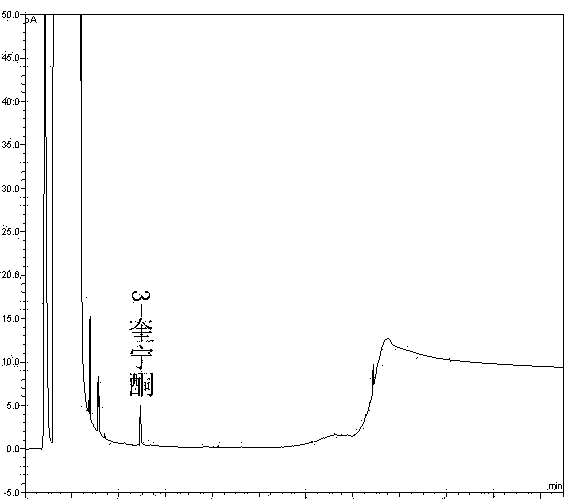
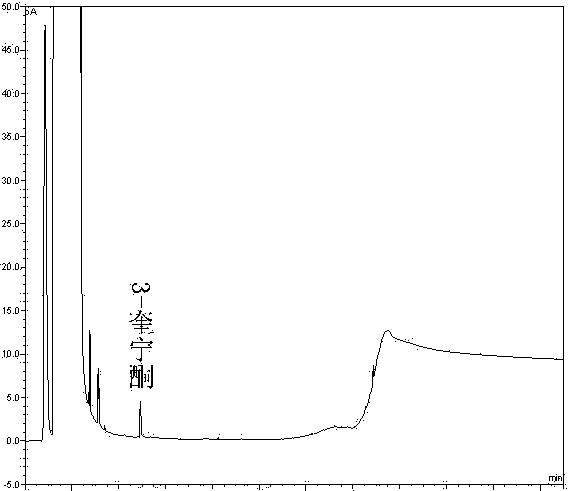
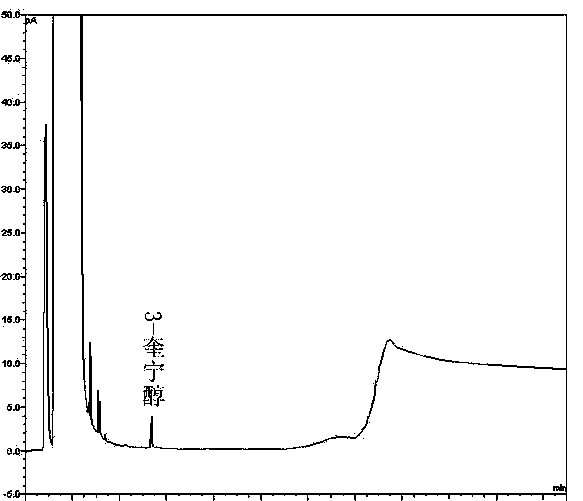
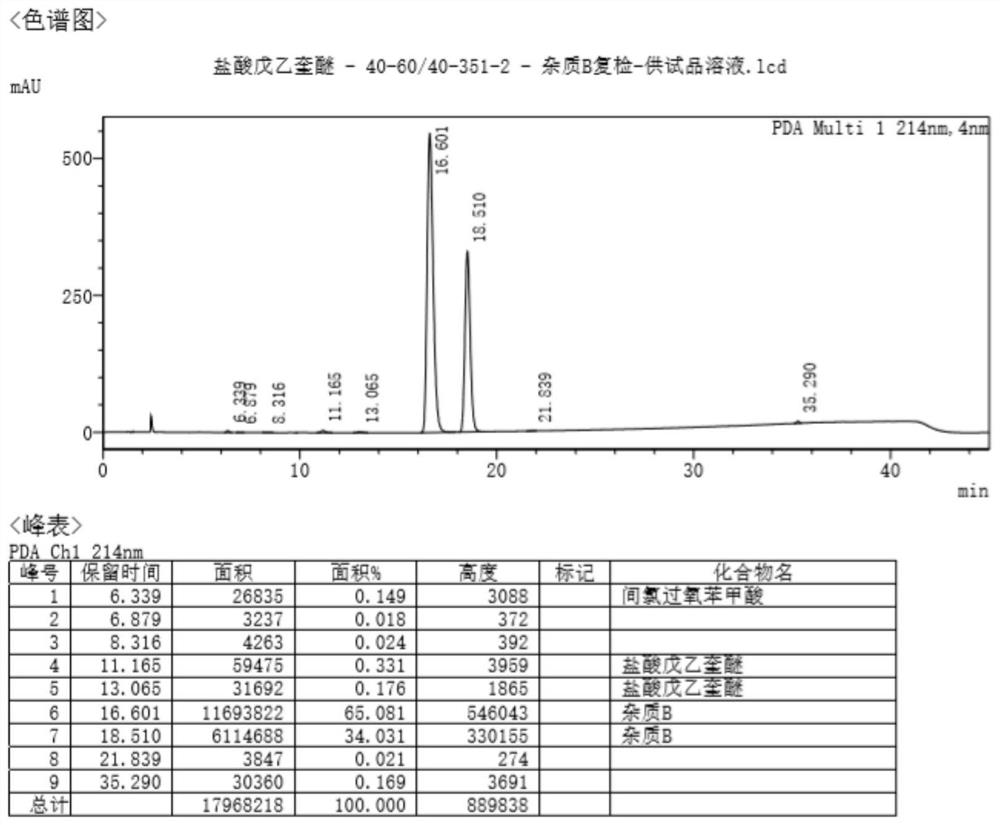
Method of detecting impurities in penehyclidine hydrochloride injection
ActiveCN104237394AImprove controllabilityDirect analysis methodComponent separationVapor phase chromatographyOrganosolv
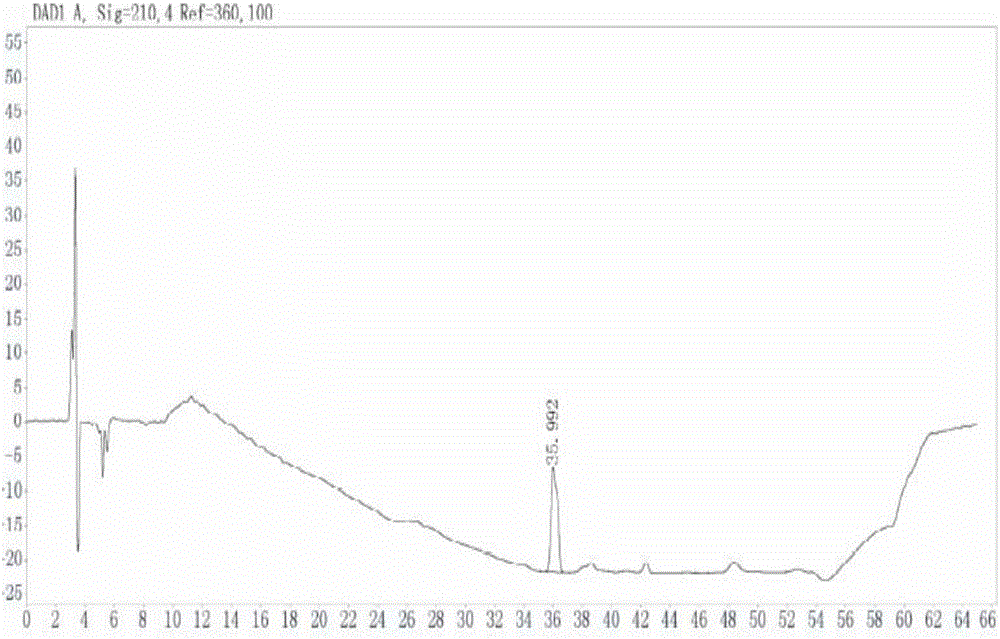
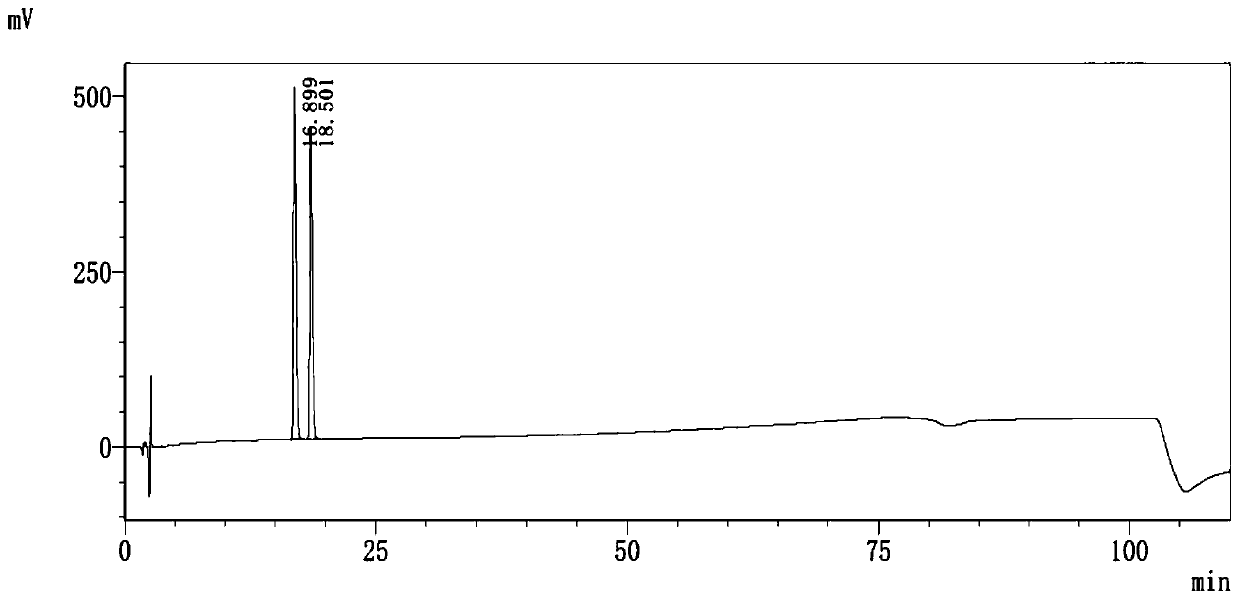
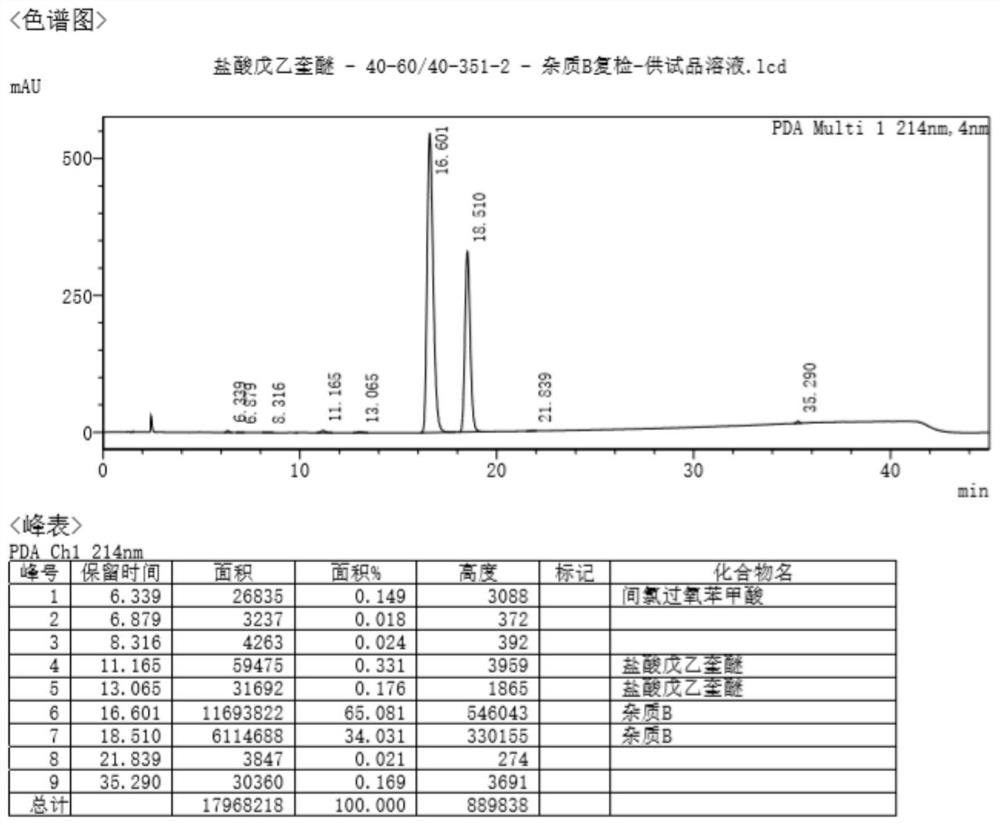
The invention discloses a method of detecting two impurities containing a quinuclidine ring in a penehyclidine hydrochloride injection. The method adopts a gas chromatographic method to detect the contents of the impurities, namely the content of 3-quinuclidone and the content of 3-quinuclidinol. During detection, a sample of the penehyclidine hydrochloride injection is subjected to vacuum rotary evaporation to remove the solvent in the injection, is dissolved with an alkaline organic solvent so as to convert all the hydrochlorides into free alkalis and is dried by blowing nitrogen, and then dimethylformamide is added to prepare a sample solution to be detected; and impurity reference substances are salified by adding an acid firstly, then is processed as the same processing manner for the sample solution to be detected, and is prepared into impurity reference substance solutions. The sample solution to be detected and the impurity reference substance solutions are directly injected respectively, chromatograms are collected, and the contents of the impurities are calculated based on peak areas by an external standard method. The method of detecting the impurities has characteristics of simple and convenient operation, high sensitivity, capability of quantitative measurement, high accuracy and good reproducibility, effectively controls the product quality of the penehyclidine hydrochloride injection, and guarantees safety and effectiveness of clinical medication.
Owner:CHONGQING XIANYANG PHARMA TECH CO LTD
Detection method for determining impurities in penehyclidine hydrochloride through high performance liquid chromatography (HPLC)
ActiveCN104297354AEasy to separateSimple and fast operationComponent separationQuantitative determinationPhosphoric acid
The invention discloses a detection method for determining two impurities in penehyclidine hydrochloride. In the method, high performance liquid chromatography (HPLC) is employed. Acetonitrile and 20 mmol / L monopotassium phosphate (containing 0.5% of triethylamine with the pH being adjusted to 3.0 through diluted phosphoric acid) are employed as a mobile phase. The contents of the impurities are determined through gradient elute. During determination, a solvent is prepared according to an initial mobile phase proportion, namely, the acetonitrile : the 20 mmol / L monopotassium phosphate (containing 0.5% of triethylamine with the pH being adjusted to 3.0 through diluted phosphoric acid) is 32:68. The penehyclidine hydrochloride is prepared into a sample solution through the solvent and an impurity referent substance solution is prepared through the same method to obtain the impurity referent substance solution. The sample solution, the impurity referent substance solution are injected directly. A chromatogram is collected and the contents of the impurities are calculated according to an external standard method according to the areas of peaks. The method is simple in operation, is high in sensitivity, can be used in quantitative determination, is high in accuracy and is good in reproducibility so that product quality of the penehyclidine hydrochloride can be effectively controlled and clinical medication is ensured to be safe and effective.
Owner:重庆科塞亚医药科技有限责任公司
Application of penehyclidine hydrochloride in preparing medicine
InactiveCN1739516AEffective for abdominal painEffective for biliary colicOrganic active ingredientsAerosol deliveryDiseaseRespiratory tract disease
The present invention relates to the application of penehyclidine hydrochloride in preparing medicine, and is especially the application of penehyclidine hydrochloride in preparing medicine for treating asthma, chronic bronchitis and other chronic obstructive respiratory tract diseases. Animal experiment and clinical observation prove the remitting effect of penehyclidine hydrochloride on asthma, chronic bronchitis and other chronic obstructive respiratory tract diseases. The present invention expands the medicinal use of penehyclidine hydrochloride.
Owner:CHENGDU LIST PHARMA
Method of detecting impurities in penehyclidine hydrochloride
ActiveCN104237393AQuantitative detection of contentImprove controllabilityComponent separationPeak area3-quinuclidinol
The invention discloses a method of detecting two impurities containing a quinuclidine ring in penehyclidine hydrochloride. The method adopts a gas chromatographic method to detect the contents of the impurities, namely the content of 3-quinuclidone and the content of 3-quinuclidinol. During detection, a sample of the penehyclidine hydrochloride is dissolved with an alkaline organic solvent so as to convert all the hydrochlorides into free alkalis and is dried by blowing nitrogen, and then dimethylformamide is added to prepare a sample solution to be detected; and impurity reference substances are processed as the same manner and prepared into impurity reference substance solutions. The sample solution to be detected and the impurity reference substance solutions are directly injected respectively, chromatograms are collected, and the contents of the impurities are calculated based on peak areas by an external standard method. The method of detecting the impurities has characteristics of simple and convenient operation, high sensitivity, capability of quantitative measurement, high accuracy and good reproducibility, effectively controls the product quality of the penehyclidine hydrochloride and guarantees safety and effectiveness of clinical medication.
Owner:重庆科塞亚医药科技有限责任公司
Preparation method of penehyclidine hydrochloride powder injection for injecting
The invention discloses a preparation method of a penehyclidine hydrochloride powder injection for injecting, which belongs to the field of medicinal supplies. The method comprises the following process steps of: dissolving a penehyclidine hydrochloride bulk pharmaceutical into a solution of absolute ethanol-absolute ether, adding an appropriate amount of active carbon for decolorizing, rough filtering for decarbonizing, and filtering with a microporous filtering film of 0.22 mum to obtain an aseptic and clear solution; in an aseptic environment, crystallizing the aseptic and clear solution of the penehyclidine hydrochloride, filtering an obtained crystal, washing, draining and drying; and detecting moisture and content, detecting aseptic state, charging into a sterilized penicillin bottle, covering with a butyl rubber plug, and rolling an aluminum cover to obtain the penehyclidine hydrochloride powder injection. Due to the adoption of the preparation method, a preparation is more stable on the aspects of pH value, relevant substances and content, the toxicity is lower, and the safety and effectiveness of the penehyclidine hydrochloride and the quality of a product are ensured more easily.
Owner:JINZHOU AHON PHARM CO LTD
Application of penehyclidine hydrochloride in preparing medicament for treating cardiogenic shock
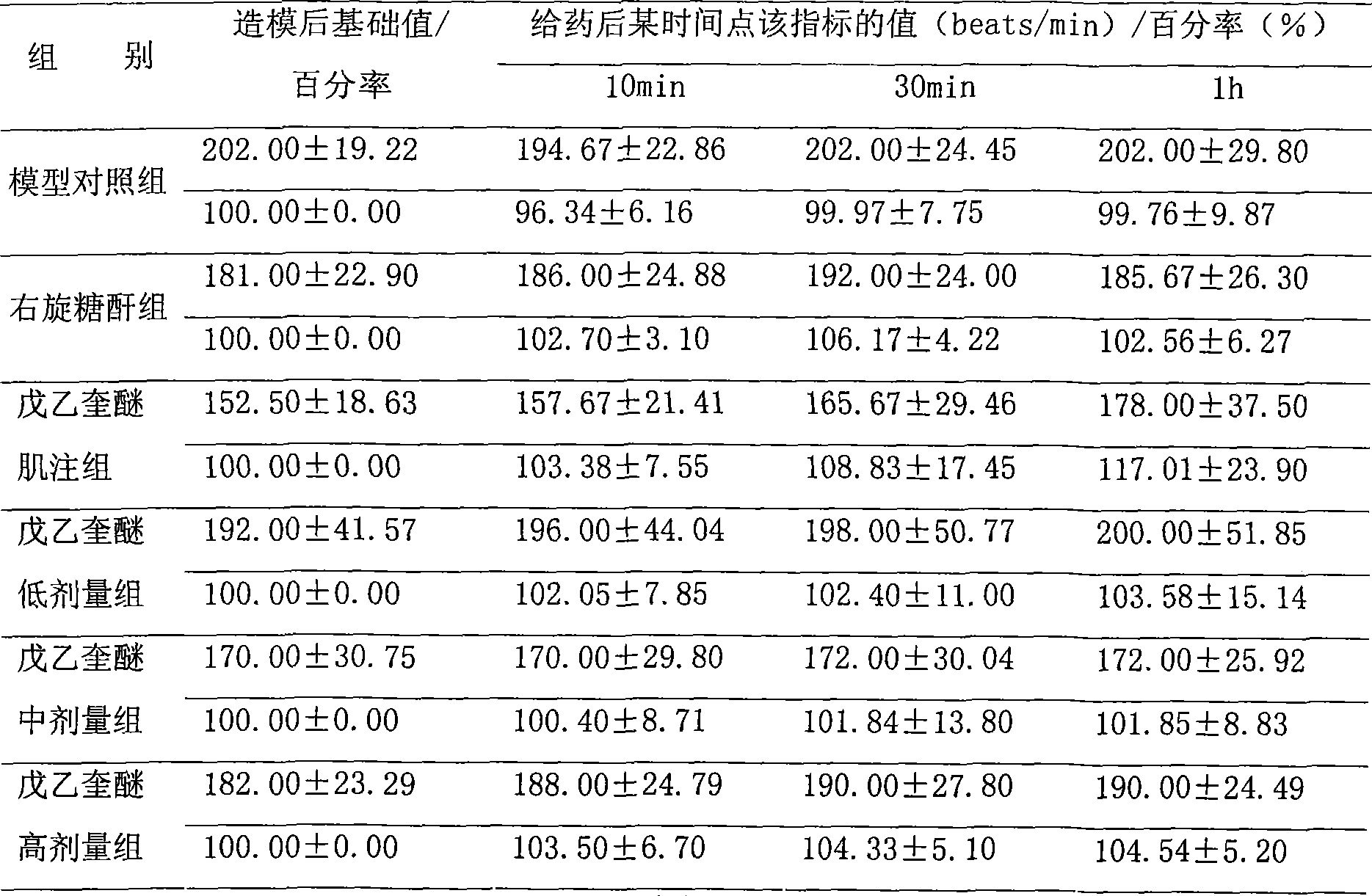
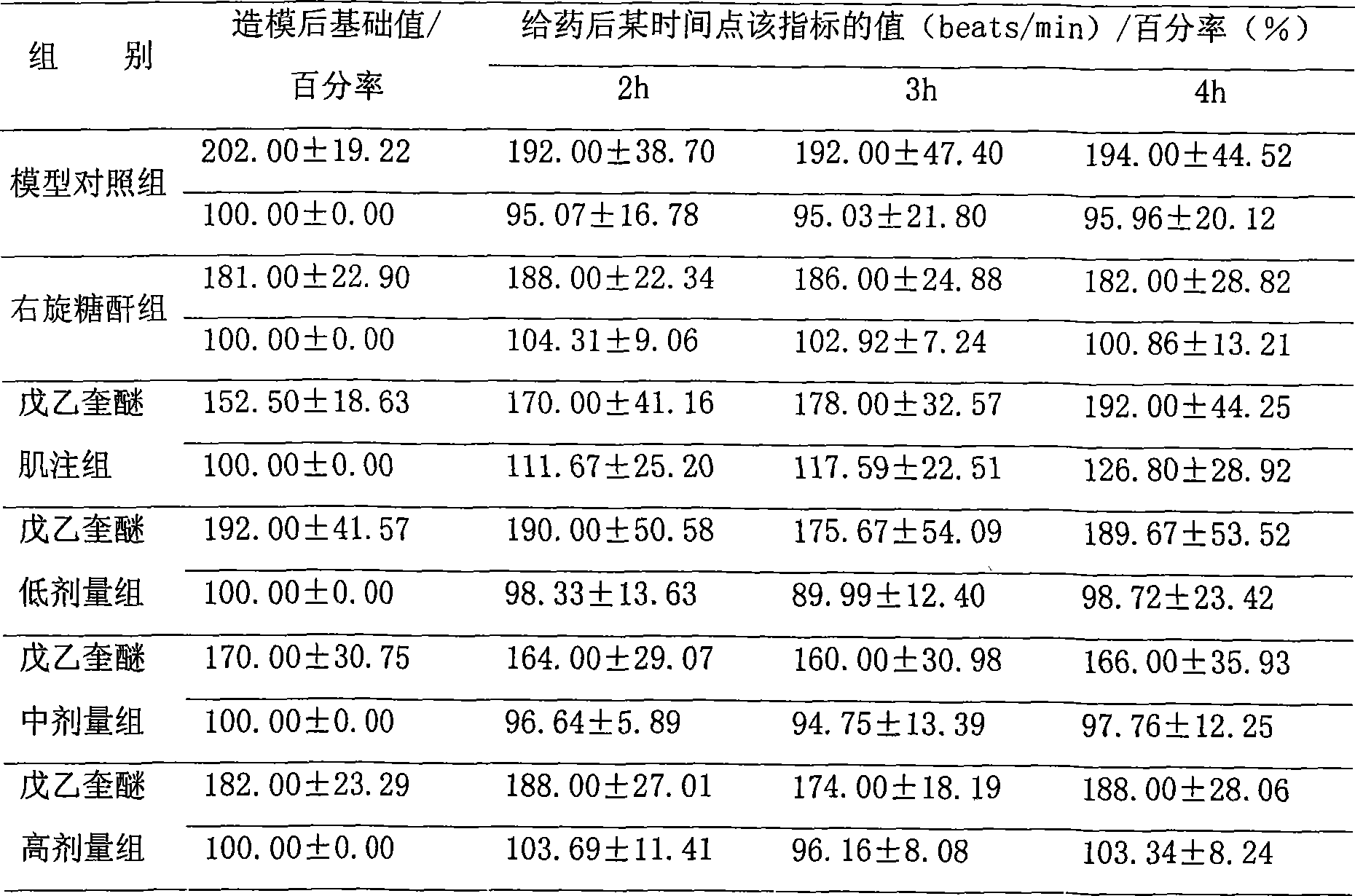
The invention relates to the use of penehyclidine hydrochloride in the preparation of medicines for treating cardiogenic shock diseases indicating that, in pathological changes, systolic pressure and mean arterial pressure decrease at least by 40%, the maximum rise speed of internal pressure of left ventricle (LV+dp / dtmax) decreases at least 80%, cardiac output decreases at least 40%. The application effect of the invention is shown by the influence of the penehyclidine hydrochloride on acute cardiogenic shock modles.
Owner:CHENGDU LIST PHARMA
Preparation method for impurities in penehyclidine hydrochloride
ActiveCN109824661AThe process steps are simpleSimple post-processingOrganic chemistryOrganic synthesisSide reaction
The invention relates to the technical field of organic synthesis, in particular to a preparation method for impurities in penehyclidine hydrochloride. The method comprises the steps that penehyclidine and alpha-phenyl-alpha-cyclopentyl-oxirane are adopted as raw materials, under the alkali compound effect, a reaction is performed to obtain 3-[2-cyclopentyl-2-phenyl-2-(2-cyclopentyl-2-hydroxyl-2-phenyl-ethyoxyl)ethyoxyl]quinuclidine free alkali, and after salt formation of hydrogen chloride, free alkali hydrochloride is obtained. The preparation method has the advantages that the technologicalsteps are simple, side reactions are few, operation is simple and convenient, post treatment is simple, raw materials are cheap and easy to obtain, and the yield is high, and the method is suitable for large-batch preparation.
Owner:山东博洛德生物科技有限公司
Method for preparing cyclopentyl phenyl acetaldehyde
InactiveCN110343036ARaise quality standardsQuality is easy to controlPreparation from heterocyclic compoundsStyrene oxideReference product
The invention discloses a method for preparing cyclopentyl phenyl acetaldehyde. The method comprises the following steps: by taking cyclopentyl phenyl ethylene oxide as a raw material, lewis acid as acatalyst, performing a reaction so as to obtain cyclopentyl phenyl acetaldehyde, specifically, dissolving cyclopentyl phenyl ethylene oxide into an organic solvent, adding lewis acid as the catalyst,performing a stirring reaction, adding water to implement a quenching reaction, further adding an extraction agent for extraction, collecting an organic phase, further adding a drying agent, performing filtration and concentration so as to obtain an oily substance, and performing column chromatography isolation on the obtained oily substance so as to obtain the cyclopentyl phenyl acetaldehyde. Byadopting the method disclosed by the invention, a reference product for qualitative and quantitative analysis can be provided for detection on penehyclidine hydrochloride, and the quality standard ofthe penehyclidine hydrochloride can be improved; the impurity cyclopentyl phenyl acetaldehyde is simple and convenient to prepare, the used raw materials are easy to obtain, and the impurity is low in preparation cost, high in purity and controllable in quality.
Owner:WUHAN DOCAN PHARMA
Process for preparing penehyclidine hydrochloride injection
The invention discloses a process for preparing a penehyclidine hydrochloride injection and belongs to the field of medicines. The specific preparation process of the injection is as follows: (1) treating inner packaging materials with acid in advance, then washing the inner packaging materials with purified water and water for injection and then pasteurizing and drying the inner packaging materials for standby application; (2) weighing penehyclidine hydrochloride based on the prescription dose, adding a defined amount of water for injection to dissolve penehyclidine hydrochloride, then adding water for injection to reach the total amount and stirring the liquid medicine uniformly; and (3) measuring the pH value and the content, filtering the liquid medicine after the pH value and the content are qualified, filling and sealing the filtrate in the inner packaging materials for standby application and carrying out pasteurization, thus obtaining the penehyclidine hydrochloride injection. The process has the following beneficial effects: not only can the pH value and content of the penehyclidine hydrochloride injection product obtained by the process be more stable, but also the product quality is more easily ensured; and meanwhile, the final product is proved to be safe and effective by animal long-term toxicity tests and pharmacodynamics tests.
Owner:JINZHOU AHON PHARM CO LTD
Method for preparing penehyclidine hydrochloride injection
Owner:JINZHOU AHON PHARM CO LTD
Application of penehyclidine hydrochloride in preparing medicine
InactiveCN1739517AEffective for abdominal painEffective for biliary colicOrganic active ingredientsAerosol deliveryCurative effectPharmacology
he present invention relates to the application of penehyclidine hydrochloride in preparing medicine, and is especially the application of penehyclidine hydrochloride in preparing medicine for treating urinary incontinence. Animal experiment and clinical observation prove the high curative effect of penehyclidine hydrochloride on urinary incontinence. The present invention expands the medicinal use of penehyclidine hydrochloride.
Owner:CHENGDU LIST PHARMA
Method for purifying 3-(2-cyclopentyl-2-hydroxy-2-phenylethoxy) quinuclidine and preparing penehyclidine hydrochloride
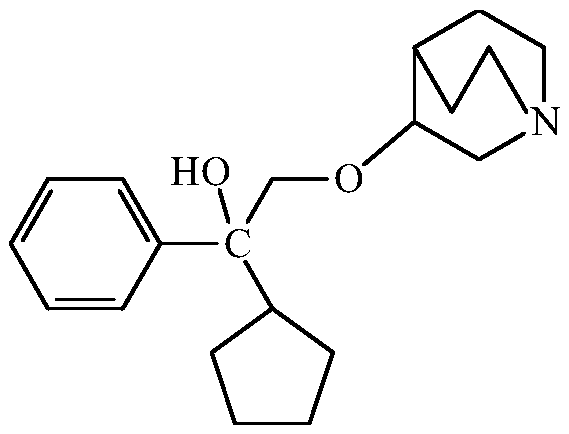
The invention provides a method for purifying 3-(2-cyclopentyl-2-hydroxy-2-phenylethoxy) quinuclidine and preparing penehyclidine hydrochloride. The method comprises the following steps: (1) mixing 3-quinuclidinol with a dimethyl sulfoxide solution, and reacting with sodium hydride and a 1,1-phenyl amyl oxirane solution; (2) adding purified water and ethyl acetate to carry out extraction, collecting the organic phase, washing, carrying out back extraction with a hydrochloric acid solution, and collecting the water layer; (3) adjusting the pH value of the water layer to 5-7, washing the water layer by ethyl acetate, adjusting the pH value to 9-12 again, extracting the water layer by ethyl acetate, and collecting the organic phase; and (4) filtering, and carrying out reduced pressure concentration to obtain 3-(2-cyclopentyl-2-hydroxy-2-phenylethoxy) quinuclidine oil. According to the method, by separating and purifying 3-(2-cyclopentyl-2-hydroxy-2-phenylethoxy) quinuclidine, the yield and quality of a target product are improved, and a penehyclidine hydrochloride finished product with a high yield and high purity is obtained through one-step salifying crystallization.
Owner:北京鑫开元医药科技有限公司
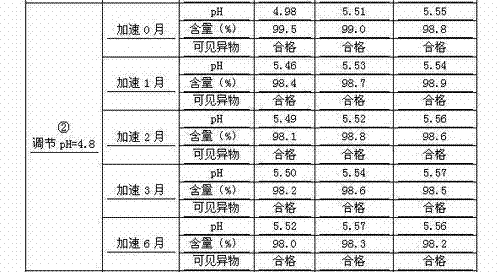
A kind of preparation method of penehyclidine hydrochloride injection
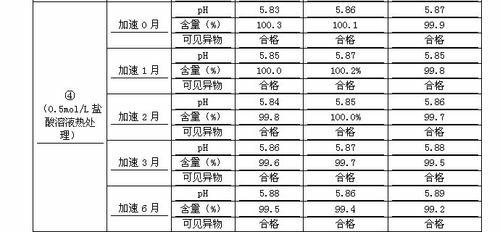
The invention discloses a novel method for preparing a penehyclidine hydrochloride injection and belongs to the field of medical drugs. The preparation process of the injection comprises the following steps: (1) performing acid treatment on an inner package in advance, washing with pure water and water for injection, performing high-temperature sterilization and drying for later use; (2) weighing penehyclidine hydrochloride according to the prescription amount, adding a proper amount of water for injection for dissolving, regulating the pH of the liquid medicine to be 4.6-4.8 by using a pH regulator, adding the water for injection to the total amount, and uniformly stirring; (3) measuring the pH value and the content, filtering and encapsulating the water in the spare inner package after the water is qualified, and sterilizing at a high temperature to obtain the injection. The penehyclidine hydrochloride injection product prepared by the process method is stable in pH and content, and the product quality is easily guaranteed; meanwhile, the long-term animal toxicity test and pharmacodynamic test prove that the final product is safe and effective.
Owner:JINZHOU AHON PHARM CO LTD
Preparation process of penehyclidine hydrochloride powder for injection
ActiveCN102579374AReduce exposureAvoid autoclavingPowder deliveryOrganic active ingredientsPenicillinFreeze-drying
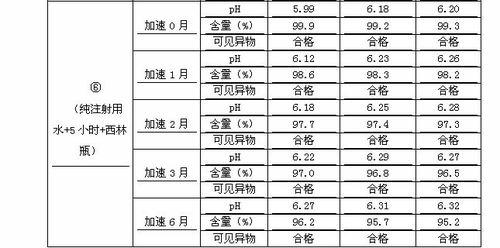
The invention discloses a preparation process of penehyclidine hydrochloride powder for injection, and belongs to the field of medical products. The process comprises the following steps: weighing penehyclidine hydrochloride and excipient; dissolving with injection water; regulating the pH value with hydrochloric acid solution; adding injection water to full dose; measuring the pH and content; filtering the liquid medicine through a 0.22mu m filter membrane, and subpackaging into aseptic penicillin bottles; and carrying out freeze drying; closing a butyl rubber stopper, and rolling an aluminum cap. The preparation prepared by the method has more stable pH value, related substances and content, and lower toxicity, so that the safety and efficiency of the penehyclidine hydrochloride and product quality are easier to be ensured.
Owner:JINZHOU AHON PHARM CO LTD
Preparation method of penehyclidine hydrochloride injection
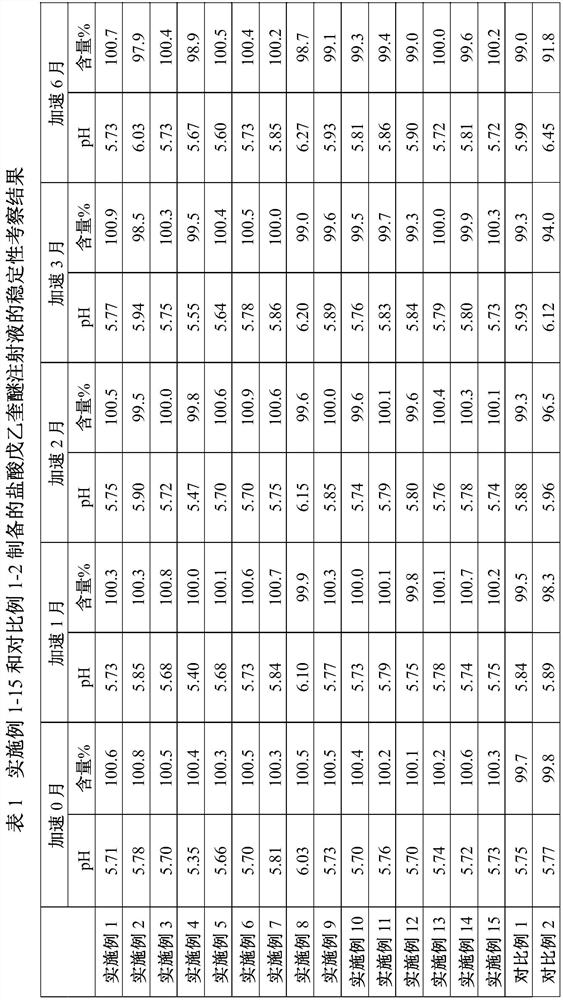
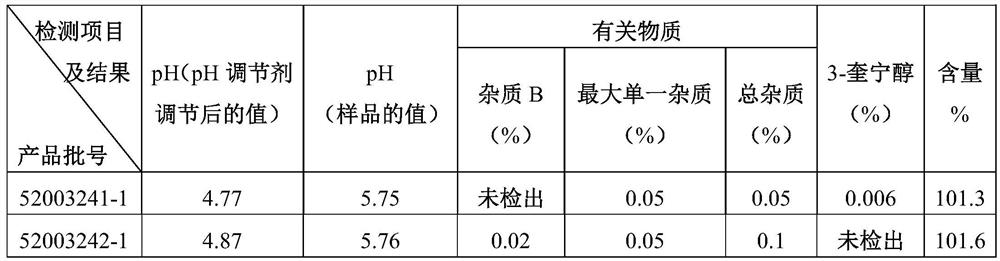
ActiveCN112716889AEnsure uniformity and stabilityQuality improvementOrganic active ingredientsPharmaceutical delivery mechanismDrug productMedicinal chemistry
The invention relates to a preparation method of penehyclidine hydrochloride injection, and belongs to the field of medical drugs. The preparation method comprises the following steps: adjusting the pH value of penehyclidine hydrochloride liquid medicine, filtering for the first time until the pH value is stable, filtering for the second time until the pH value is stable, filling and sealing, and sterilizing at high temperature to obtain the penehyclidine hydrochloride injection. According to the preparation method of the penehyclidine hydrochloride injection, provided by the invention, the pH of the penehyclidine hydrochloride liquid medicine is adjusted to 4.5-5.0, and then the liquid medicine is subjected to circulating filtration treatment through the filter elements connected in series, so that the uniformity and stability of the liquid medicine are guaranteed, and the prepared penehyclidine hydrochloride injection has better stability and is more suitable for industrial production. Moreover, when the penehyclidine hydrochloride injection prepared by the invention is placed for a long time, the pH and the content of the penehyclidine hydrochloride injection can be stably kept within a qualified range.
Owner:WUHAN DOCAN PHARMA
Application of penehyclidine hydrochloride in the treatment of infectious toxic shock with microcirculation disturbance after comprehensive treatment measures such as blood volume supplementation
The invention discloses the application of penehyclidine hydrochloride in the treatment of septic shock in which microcirculation disturbance still exists after comprehensive treatment measures such as replenishing blood volume. Infection toxic shock referred to in the present invention refers to the shock caused by bacterial attack, and the effect of the present invention is proved by the effect of penhyclidine hydrochloride aqueous solution on microcirculation disorder caused by infectious toxic shock in rabbits.
Owner:JINZHOU AHON PHARM CO LTD
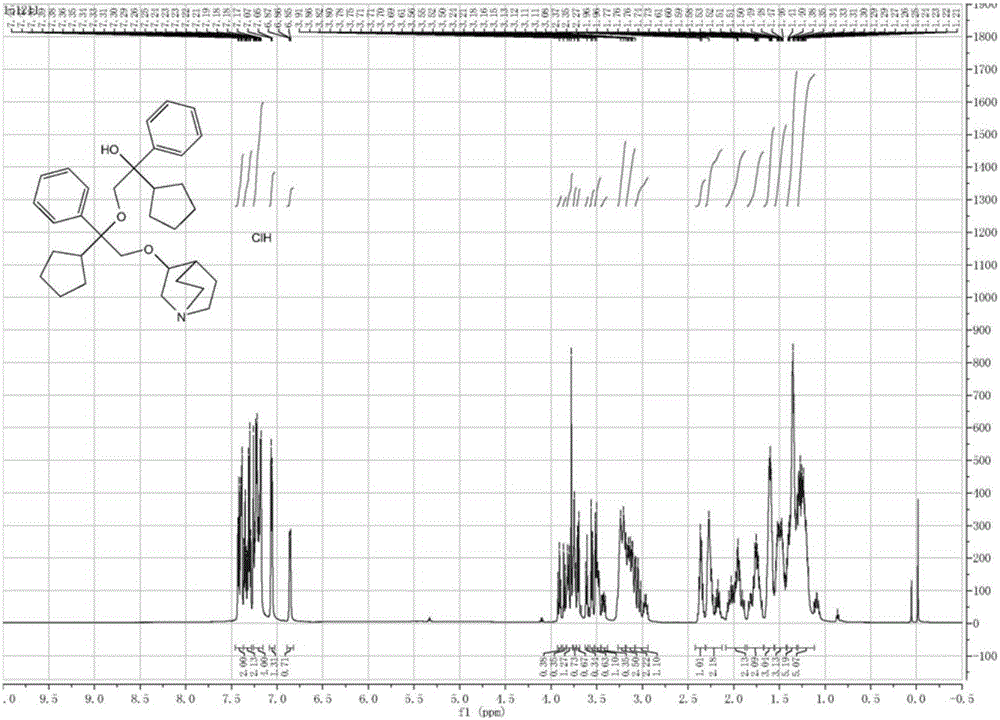
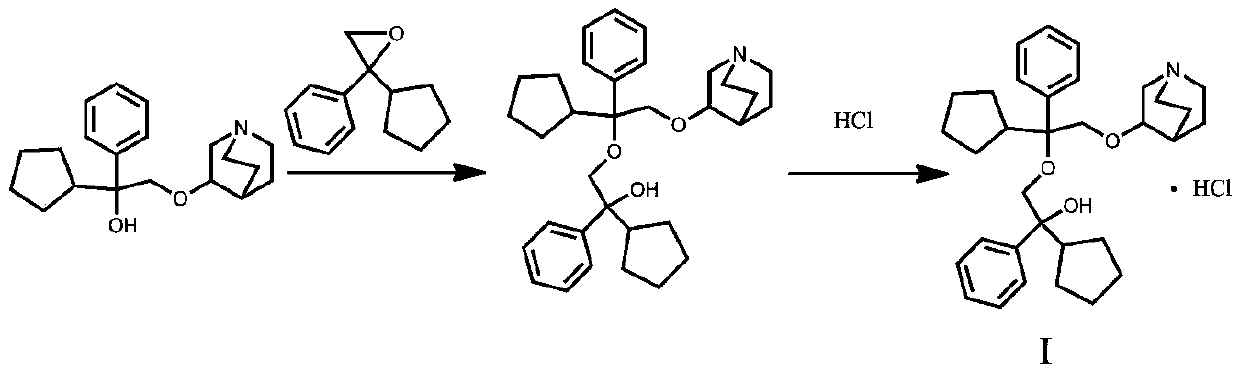
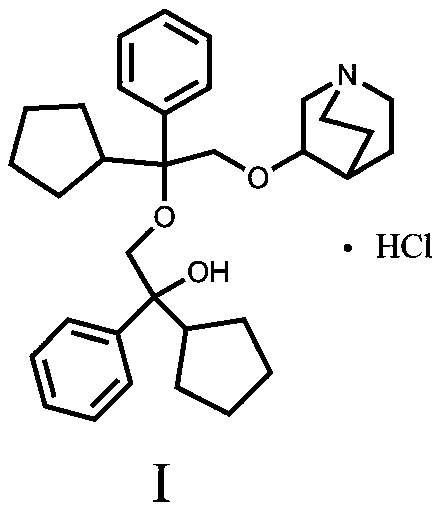
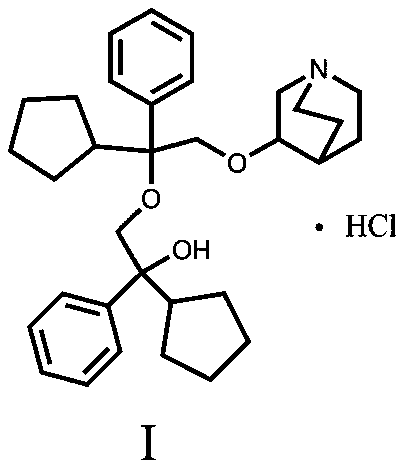
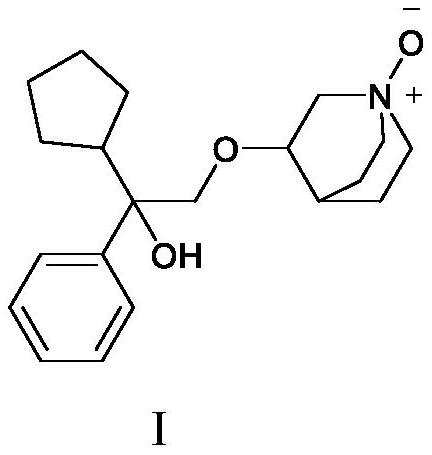
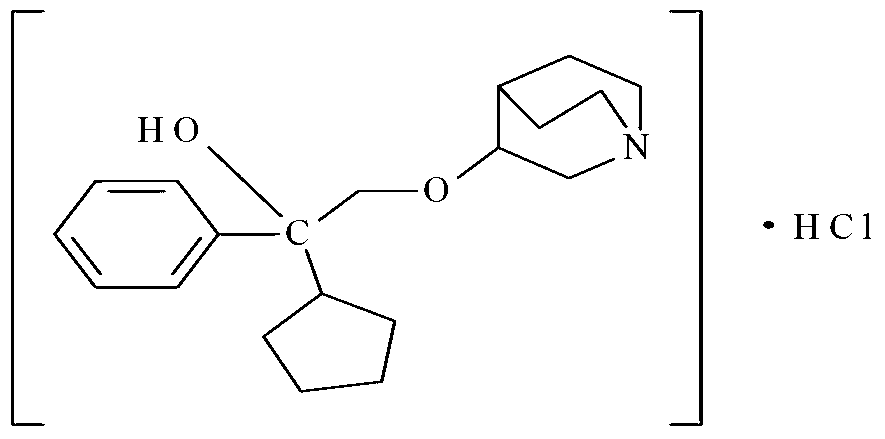
A kind of penehyclidine hydrochloride impurity and preparation method thereof
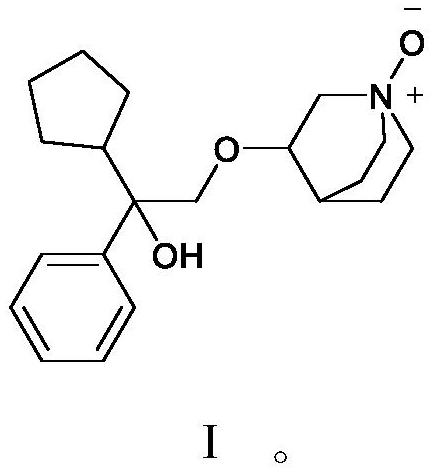
ActiveCN112028887BRaise quality standardsEnsure medication safetyOrganic chemistryEthoxidinePhysical chemistry
The invention relates to a penhyclidine hydrochloride impurity and a preparation method thereof, belonging to the technical field of medicine. The penehyclidine impurity provided by the present invention is shown in formula I, and the impurity reference substance 3-(2-cyclopentyl-2-hydroxyl-2-phenylethoxy) quinine-1-oxide synthesis method provided , the synthetic raw materials are easy to obtain, the operation is simple and convenient, the cost is low, and it has good economic value.
Owner:NHWA PHARMA CORPORATION
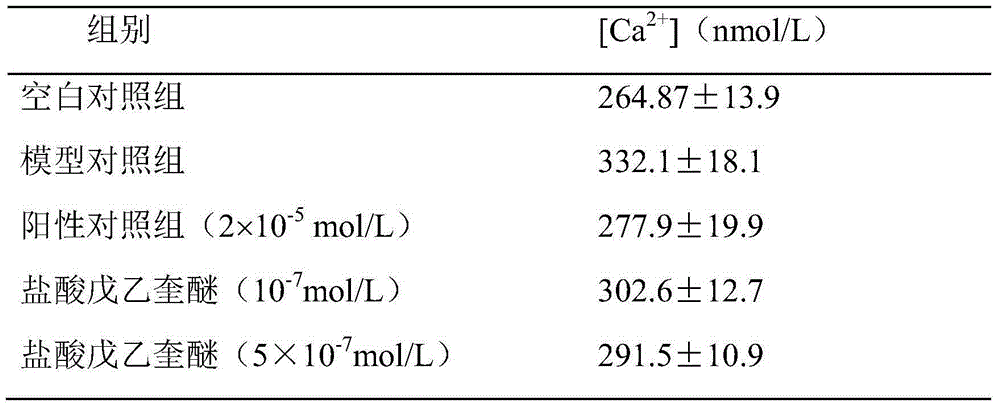
Evaluation method of effect of penehyclidine hydrochloride on calcium ion in animal model of dysmenorrhea
The invention discloses an evaluation method of influences of penehyclidine hydrochloride to calcium ions in dysmenorrhea animal model cells. The method comprises the following steps: obtaining a blank cell suspension; adding oxytocin into the blank cell suspension, and mixing for acting for 5 to 15 minutes so as to obtain an animal model cell suspension; dividing the animal model cell suspension into three groups, and adding reagents in equal volume into three groups of animal model cell suspension, wherein one group is a positive control group in which a contrast reagent is added and acts for 10 to 20 minutes, one group is an experimental group in which penehyclidine hydrochloride in different doses is added and acts for the same time with the positive control group, and one group is a model group without adding the reagent; and contrasting differences of the concentration of the calcium ions in the cells of the experimental group with the concentration of the calcium ions in the cells of the model group and the positive control group. The embodiment of the invention can reveal the influences of penehyclidine hydrochloride to the concentration of the calcium ions in the dysmenorrhea animal model cells, and can ensure that the efficacy of penehyclidine hydrochloride in different concentrations is evaluated by contrasting the concentration of the calcium ions in the cells of the experimental group with the concentration of the calcium ions in the cells of the positive control group and the model group.
Owner:SICHUAN PROVINCIAL PEOPLES HOSPITAL
A kind of detection method of impurity in penhyclidine hydrochloride determined by high performance liquid chromatography
ActiveCN104297354BEasy to detectImprove controllabilityComponent separationQuantitative determinationPhosphoric acid
The invention discloses a detection method for determining two impurities in penehyclidine hydrochloride. In the method, high performance liquid chromatography (HPLC) is employed. Acetonitrile and 20 mmol / L monopotassium phosphate (containing 0.5% of triethylamine with the pH being adjusted to 3.0 through diluted phosphoric acid) are employed as a mobile phase. The contents of the impurities are determined through gradient elute. During determination, a solvent is prepared according to an initial mobile phase proportion, namely, the acetonitrile : the 20 mmol / L monopotassium phosphate (containing 0.5% of triethylamine with the pH being adjusted to 3.0 through diluted phosphoric acid) is 32:68. The penehyclidine hydrochloride is prepared into a sample solution through the solvent and an impurity referent substance solution is prepared through the same method to obtain the impurity referent substance solution. The sample solution, the impurity referent substance solution are injected directly. A chromatogram is collected and the contents of the impurities are calculated according to an external standard method according to the areas of peaks. The method is simple in operation, is high in sensitivity, can be used in quantitative determination, is high in accuracy and is good in reproducibility so that product quality of the penehyclidine hydrochloride can be effectively controlled and clinical medication is ensured to be safe and effective.
Owner:重庆科塞亚医药科技有限责任公司
Industrial production method of penehyclidine hydrochloride injection
ActiveCN103271872AGuaranteed stabilityImprove controllabilityOrganic active ingredientsNervous disorderClinical efficacyMedicine
The invention provides an industrial production method of penehyclidine hydrochloride injection, wherein the penehyclidine hydrochloride injection is prepared by steps of adjusting pH to be not less than 4.0 and less than 4.6 by using an acid, filling and sterilizing. The industrial production method of penehyclidine hydrochloride injection provided by the invention is strong in industrialization operability; and the obtained injection is good in stability, and capable of effectively avoiding the quality changes of pH, related substances, content, related indexes and the like after being stored for a long time without the need of special storage conditions, thus bringing an effective guarantee for the safety and the clinical efficacy of the product.
Owner:CHENGDU ZIHAO PHARMA
Penehyclidine hydrochloride impurity and preparation method thereof
ActiveCN112028887ARaise quality standardsEnsure medication safetyOrganic chemistryPhysical chemistryPhenyl group
The invention relates to a penehyclidine hydrochloride impurity and a preparation method thereof, and belongs to the technical field of medicines. The penehyclidine impurity provided by the inventionis as shown in a formula I. The synthesis method of the impurity reference substance 3-(2-cyclopentyl-2-hydroxy-2-phenylethoxy)quinine-1-oxide provided by the invention has the advantages of easily available synthesis raw materials, simple and convenient operation, low cost and good economic value.
Owner:NHWA PHARMA CORPORATION
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com