Preparation method for impurities in penehyclidine hydrochloride
A technology for penehyclidine hydrochloride and penehyclidine hydrochloride is applied in the field of preparation of impurities in penehyclidine hydrochloride, can solve the problems of incomplete reaction, complicated operation, many side reactions, etc., and achieves easy operation and simple process steps. , the effect of less side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
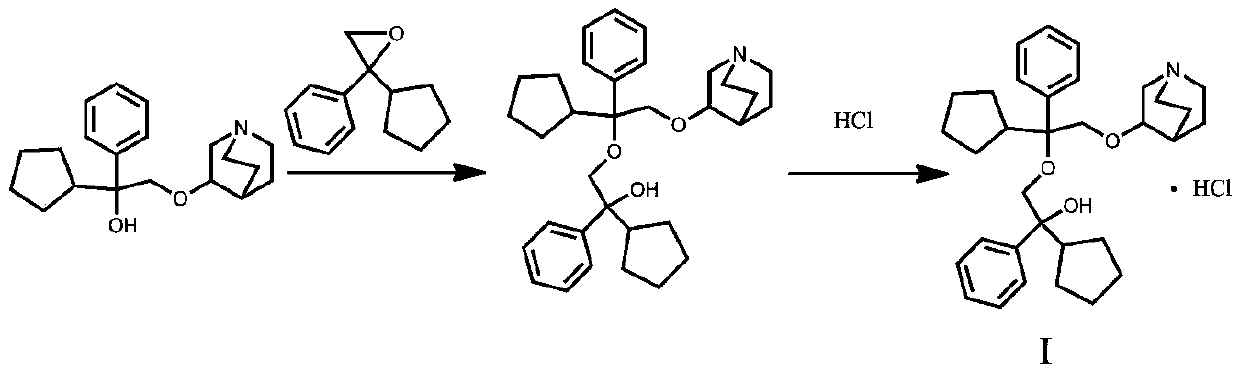
[0022] 3-[2-Cyclopentyl-2-phenyl-2-(2-cyclopentyl-2-hydroxy-2-phenyl-ethoxy)ethoxy]quinuclidane free base
[0023] Add 18.90g (0.06mol) of penehyclidine into a 500ml three-neck flask, add 150mL of DMSO, heat to 60°C, add 2.40g (0.06mol, 1eq) of NaH with a content of 60% after dissolving, and continue heating at this temperature after adding After reacting for 1 hour, add 40 mL of DMSO solution of 11.80 g (0.063 mol, 1.05 eq) of α-phenyl-α-cyclopentyl-oxirane into it, continue heating and reacting at this temperature for 6 hours, cool down to 10°C, add 100ml of water was extracted three times with methyl tert-butyl ether, the organic phases were combined, dried over anhydrous sodium sulfate, and concentrated to dryness under reduced pressure to obtain 27.41 g of light yellow viscous oil.
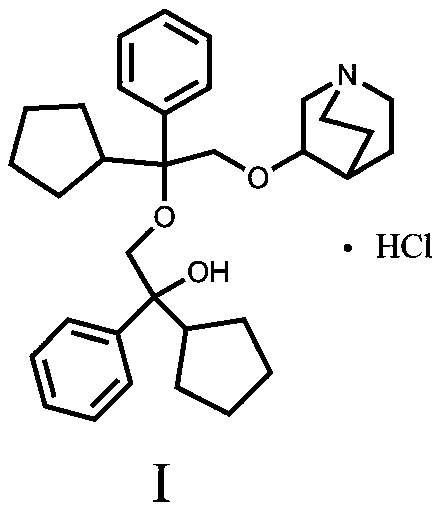
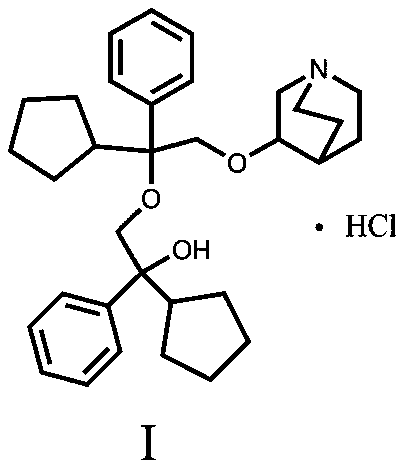
[0024] 3-[2-Cyclopentyl-2-phenyl-2-(2-cyclopentyl-2-hydroxy-2-phenyl-ethoxy)ethoxy]quinuclidine hydrochloride
[0025] 27.00 g of 3-[2-cyclopentyl-2-phenyl-2-(2-cyclopentyl-2-hydroxy-2-pheny...
Embodiment 2
[0027] 3-[2-Cyclopentyl-2-phenyl-2-(2-cyclopentyl-2-hydroxy-2-phenyl-ethoxy)ethoxy]quinuclidane free base
[0028] Add 18.90g (0.06mol) of penehyclidine into a 500ml three-necked flask, add 150mL of DMSO, heat to 60°C, add 2.52g (0.063mol, 1.05eq) of NaH with a content of 60% after the addition is complete, and continue at this temperature After heating and reacting for 1 hour, add 11.80g (0.063mol, 1.05eq) of α-phenyl-α-cyclopentyl-oxirane in 40mL of DMSO solution, continue heating and reacting at this temperature for 4 hours, then lower the temperature to 10°C, Add 100 ml of water, extract three times with methyl tert-butyl ether, combine the organic phases, dry over anhydrous sodium sulfate, and concentrate to dryness under reduced pressure to obtain 27.86 g of light yellow viscous oil.
[0029] 3-[2-Cyclopentyl-2-phenyl-2-(2-cyclopentyl-2-hydroxy-2-phenyl-ethoxy)ethoxy]quinuclidine hydrochloride
[0030] Add 27.00 g of 3-[2-cyclopentyl-2-phenyl-2-(2-cyclopentyl-2-hydroxy-...
Embodiment 3
[0032] Add 18.90g (0.06mol) of penehyclidine into a 500ml three-necked flask, add 150mL of DMSO, heat to 60°C, add 2.52g (0.063mol, 1.05eq) of NaH with a content of 60% after the addition is complete, and continue at this temperature After heating and reacting for 1 hour, add 12.36g (0.066mol, 1.1eq) of α-phenyl-α-cyclopentyl-oxirane in 40mL of DMSO solution, continue heating and reacting at this temperature for 4 hours, then lower the temperature to 10°C, Add 100ml of water, extract three times with methyl tert-butyl ether, combine the organic phases, dry over anhydrous sodium sulfate, and concentrate to dryness under reduced pressure to obtain 28.12 g of light yellow viscous oil.
[0033] 3-[2-Cyclopentyl-2-phenyl-2-(2-cyclopentyl-2-hydroxy-2-phenyl-ethoxy)ethoxy]quinuclidine hydrochloride
[0034] Add 27.50 g of 3-[2-cyclopentyl-2-phenyl-2-(2-cyclopentyl-2-hydroxy-2-phenyl-ethoxy)ethoxy]quinuclidane free base to 15 ml Methanol and 135ml of ethyl acetate, stirred and mixed ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com