Preparation method of impurity in penehyclidine hydrochloride
A technology for penehyclidine hydrochloride and impurities, applied in the field of medicine, can solve problems such as small amount, complicated separation operation, etc., and achieve the effects of reducing the complexity of operation and increasing the content of impurities
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
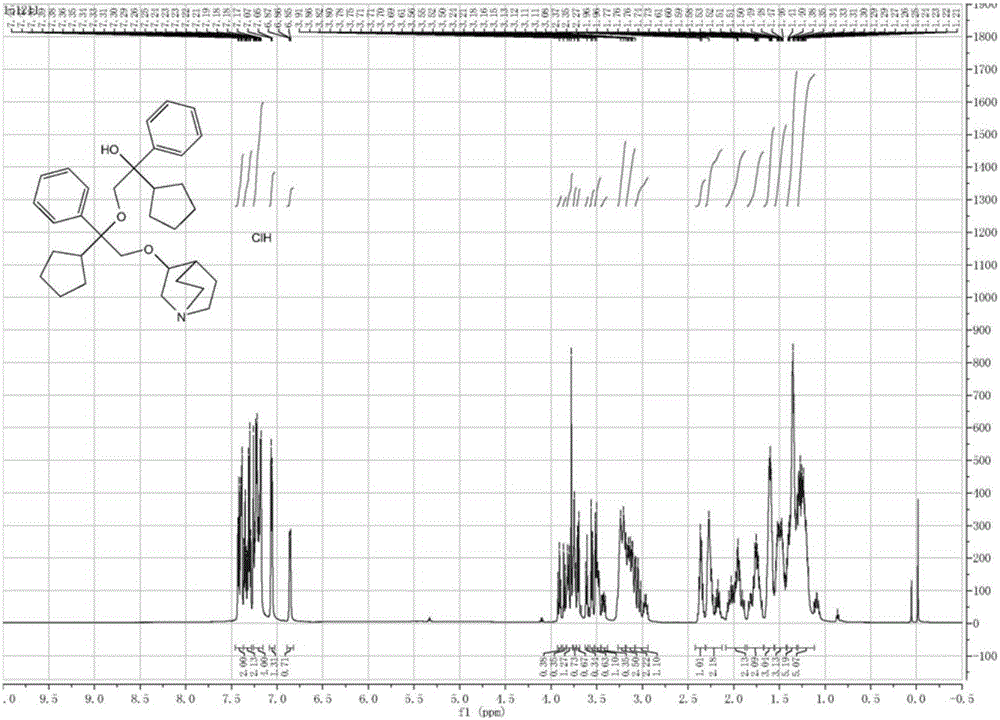
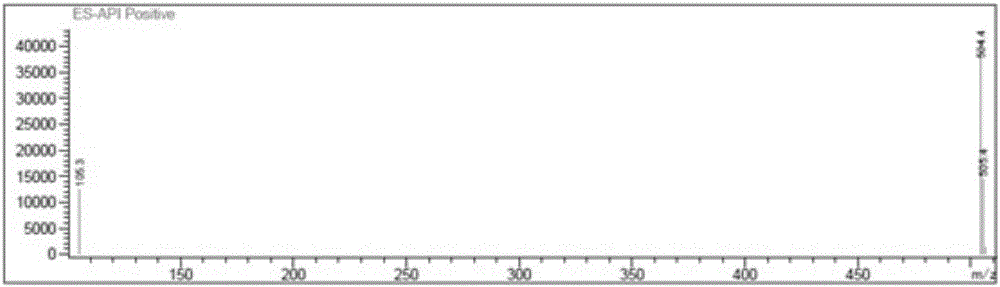
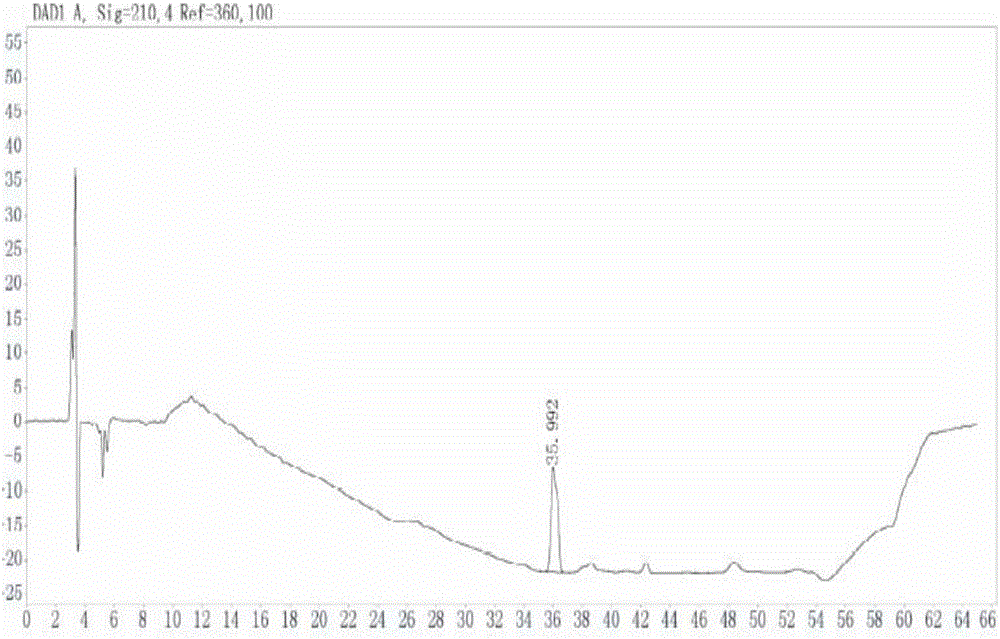
[0048] A preparation method for impurities in penhyclidine hydrochloride, comprising the following steps:
[0049] (1) Add 100ml dimethyl sulfoxide and 20g 3-quinine alcohol into a 1L reaction flask, stir to dissolve the solid, add 7.5g sodium hydride (60%) in portions, stir and mix, heat up to 60°C and stir for 1 hours, in 3-quinine alcohol sodium salt. Warming up to 70°C, 113.4g dimethyl sulfoxide solution (100ml) of α-phenyl-α-cyclopentyl-α-hydroxy ethyl p-toluenesulfonate was added dropwise to the mixture, the temperature was maintained and the reaction was stirred for 6 hours, and the temperature was lowered to Below 10°C, add 150ml of purified water dropwise, after dropping, add 300ml of ethyl acetate, stir evenly, and then let stand to separate the liquid.
[0050] (2) The organic phase was extracted with 300ml 10% citric acid aqueous solution, and the organic phase was separated, and the aqueous phase was adjusted to pH 8-9 with saturated sodium bicarbonate solution, ...
Embodiment 2
[0058] A preparation method for impurities in penhyclidine hydrochloride, comprising the following steps:
[0059] (1) Add 120ml of dimethyl sulfoxide and 24g of 3-quinine alcohol into a 2L reaction flask, stir to dissolve the solid, add 9.8g of sodium hydride (60%) in portions, stir and mix, heat up to 50°C and stir for 1 hour , in 3-quinine alcohol sodium salt. Warming up to 70°C, 204.2g of dimethyl sulfoxide solution (200ml) of α-phenyl-α-cyclopentyl-α-hydroxy ethyl p-toluenesulfonate was added dropwise to the mixture, the temperature was maintained and the reaction was stirred for 8 hours, and the temperature was lowered to Below 10°C, add 300ml of purified water dropwise, after dropping, add 400ml of ethyl acetate, stir evenly, then let stand to separate the liquid.
[0060] (2) The organic phase was extracted with 370 ml of 10% citric acid aqueous solution, and the organic phase was separated. The aqueous phase was adjusted to pH 8-9 with saturated sodium bicarbonate s...
Embodiment 3
[0066] A preparation method for impurities in penhyclidine hydrochloride, comprising the following steps:
[0067] (1) Add 60ml dimethyl sulfoxide and 12.7g 3-quinuclidinol to a 1L reaction flask, stir to dissolve the solid, add 5g sodium hydride (60%) in portions, stir and mix, heat up to 50°C and stir for 1 hours, in 3-quinine alcohol sodium salt. The temperature was raised to 80° C., 90 g of dimethyl sulfoxide solution (90 ml) of ethyl α-phenyl-α-cyclopentyl-α-hydroxy p-toluenesulfonate was added dropwise, and the temperature was maintained and the reaction was stirred for 7 hours. Cool down to below 10°C, add 100ml of purified water dropwise, add 200ml of ethyl acetate, stir evenly, and then let stand to separate the liquid.
[0068] (2) The organic layer was extracted with 230 ml of 10% citric acid aqueous solution, and the organic layer was separated. The aqueous layer was adjusted to pH 8-9 with saturated sodium bicarbonate solution, and 100ml of ethyl acetate was add...
PUM
| Property | Measurement | Unit |
|---|---|---|
| The inside diameter of | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com