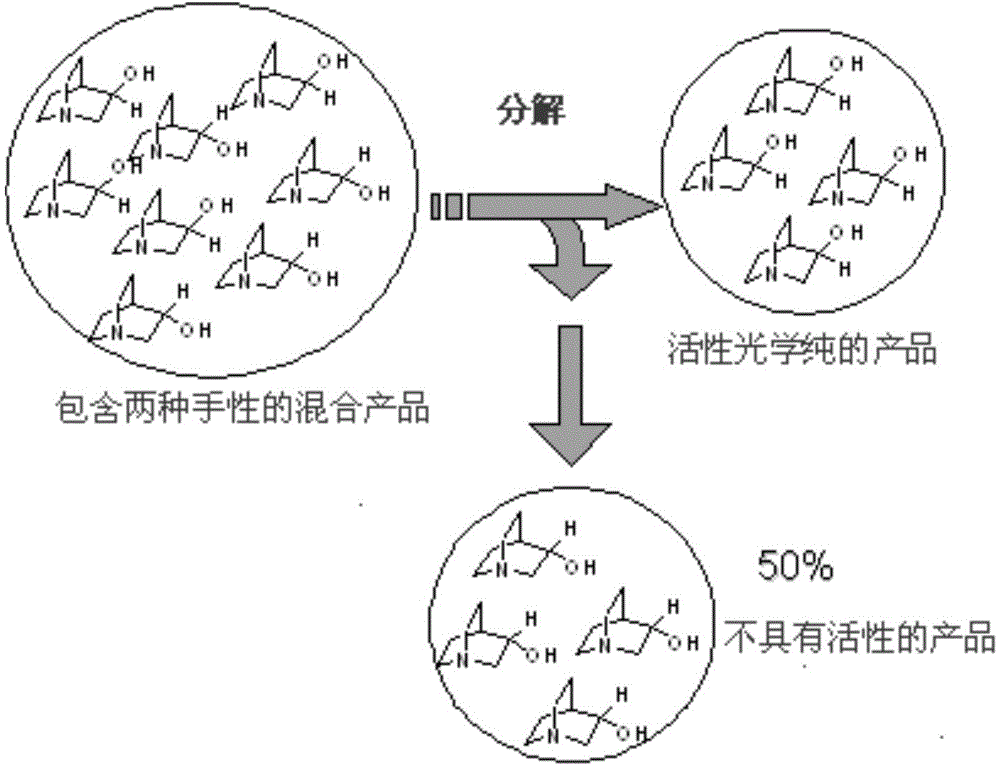
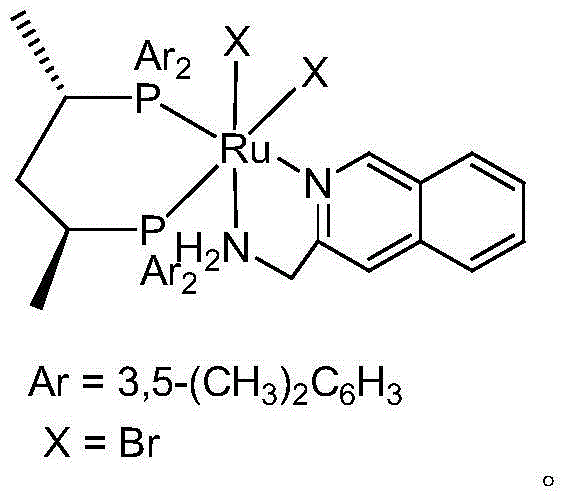
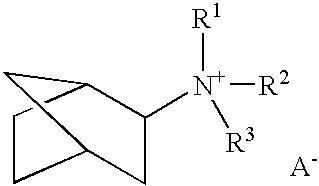
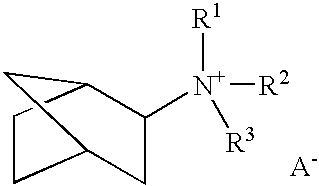
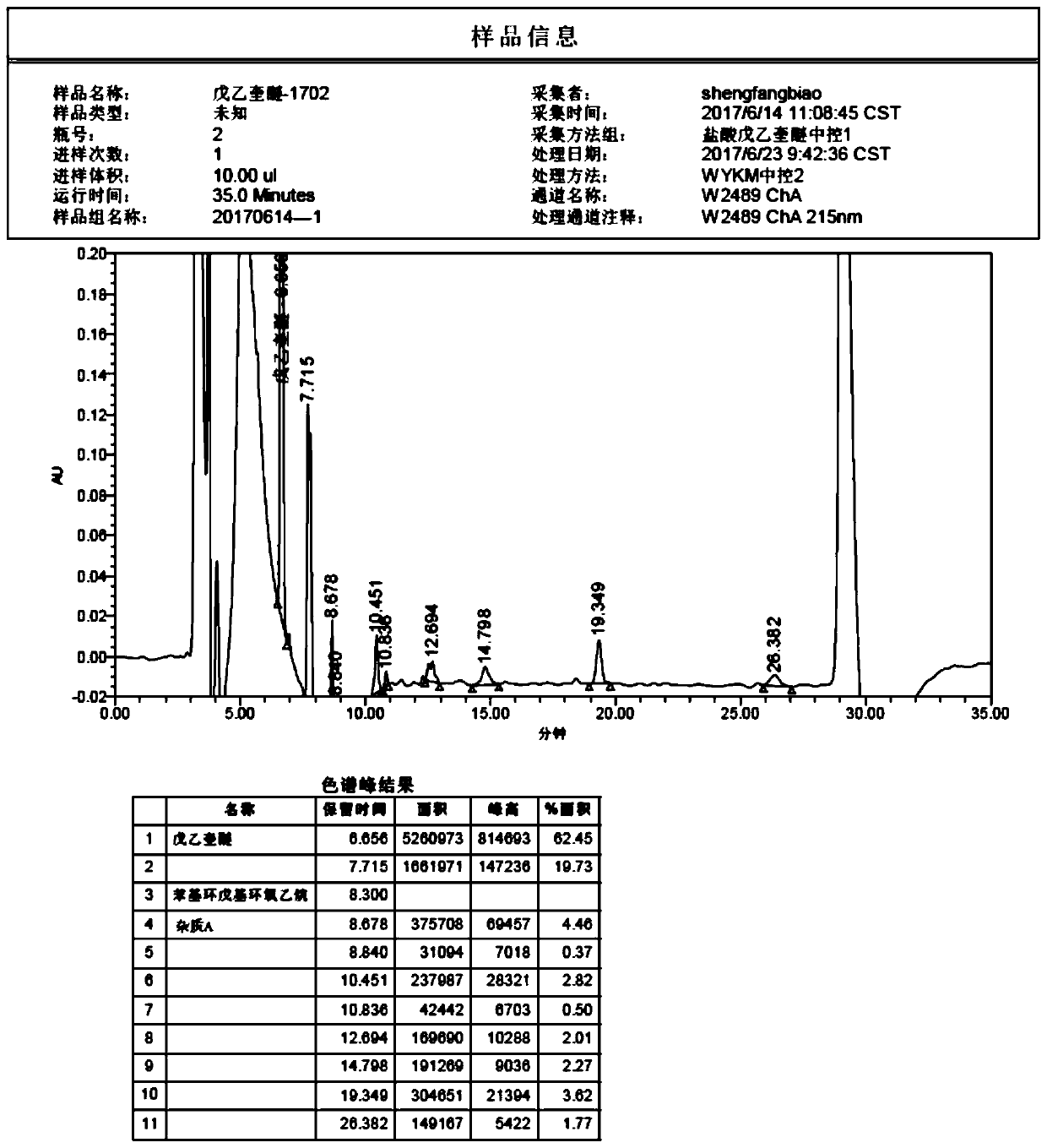
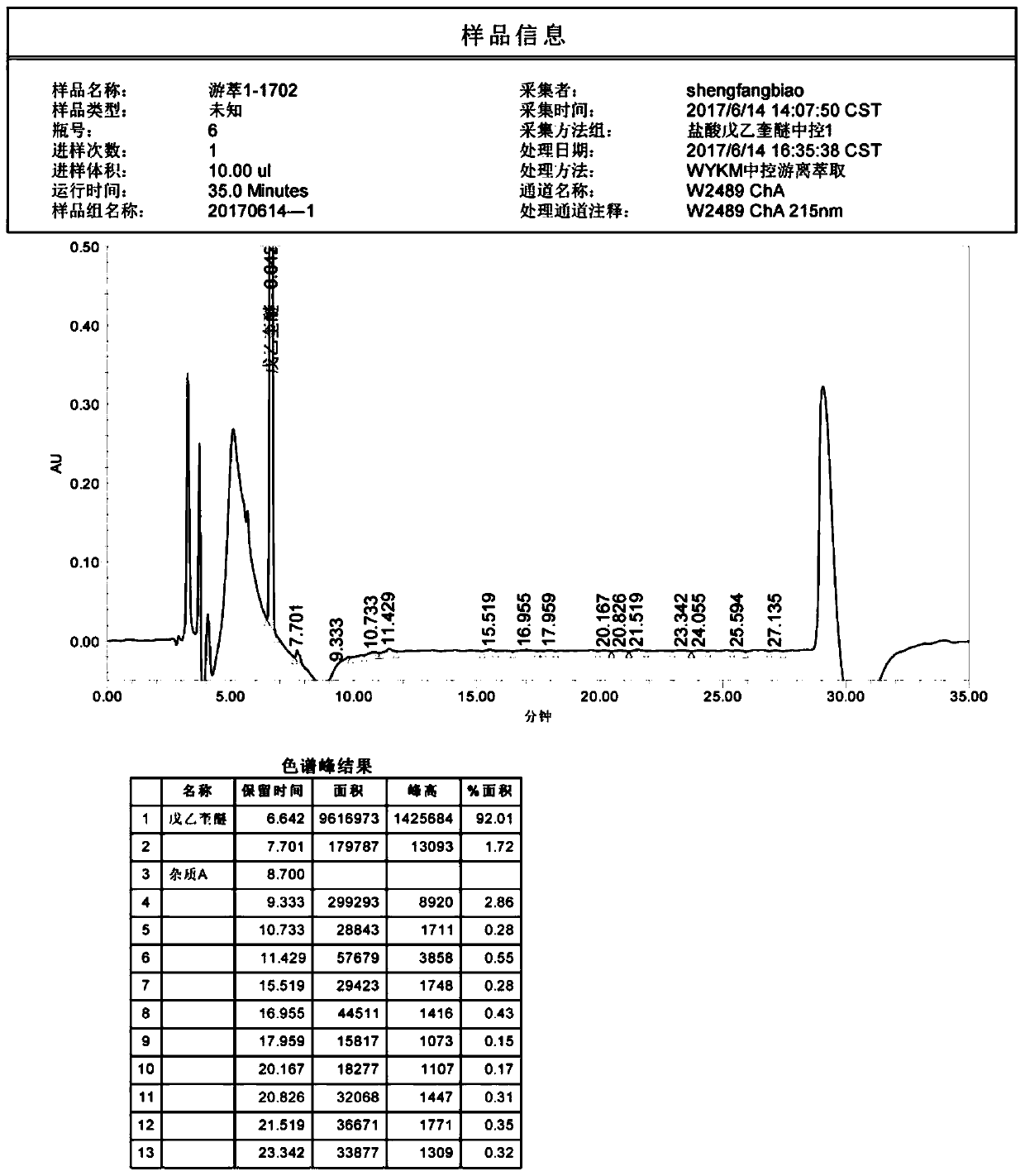
Patents
Literature
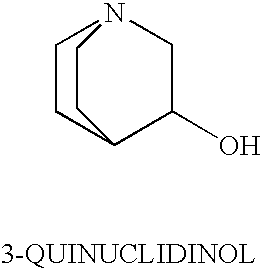
54 results about "3-quinuclidinol" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
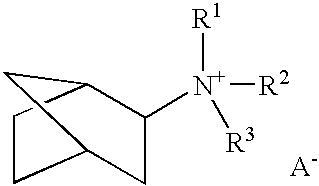
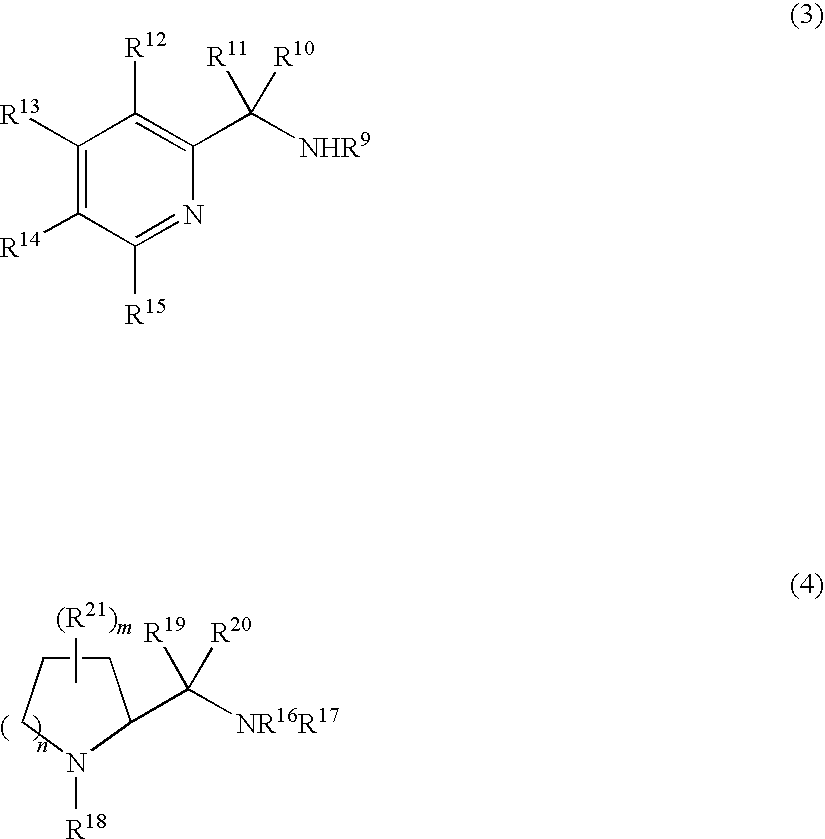
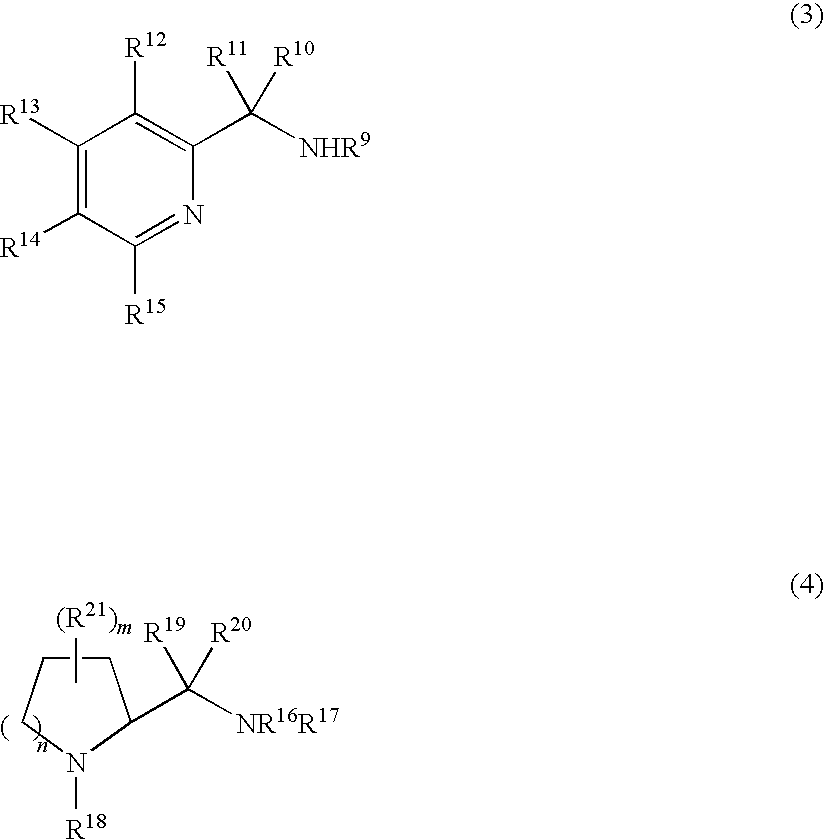
3-Quinuclidinol has been used: • as chiral building block for many antimuscarinic agents • in chemoselective α−iodination of various simple and multi-functionalised acrylic esters via Morita-Baylis-Hillman protocol • as reagent for cleavage of β-keto and vinylogous β-keto esters
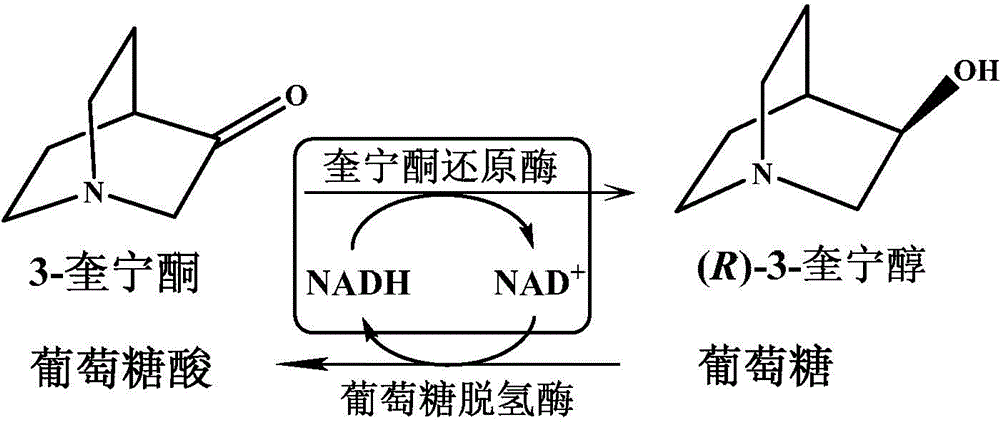
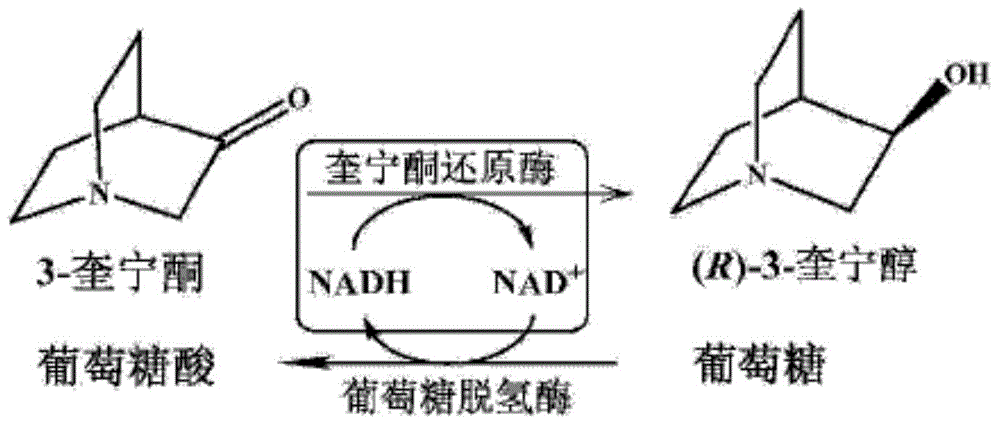
Quininone reductase and application thereof to asymmetric synthesis of (R)-3-quinuclidinol
ActiveCN103555608AIncrease concentrationMild reaction conditionsBacteriaMicroorganism based processesAnticholinergic DrugsEnantioselective synthesis
The invention discloses anagrobacterium radiobacter, a quininone reductase expressed thereby and a gene thereof, recombinant expression plasmid containing the gene, recombinant expression vector containing the gene, a recombinase of quininone, a preparation method of the recombinase , and application of the recombinase to asymmetric reduction of 3-quininone as a catalyst for preparation of (R)-3-quinuclidinol. Compared with other preparation methods for (R)-3-quinuclidinol, the (R)-3-quinuclidinol prepared by employing the quininone reductase provided by the invention is not only high in product concentration, but also good in optical purity; the reaction condition is mild, the operation is convenient, and the preparation is easy to amplify; and therefore the application of the quininone reductase to asymmetric synthesis of (R)-3-quinuclidinol has extremely good industrial application prospect in the production of intermediates of anticholinergic drugs.
Owner:EAST CHINA UNIV OF SCI & TECH
Oxygenate conversion using boron-containing molecular sieve CHA
InactiveUS20060116541A1Hydrocarbon from oxygen organic compoundsEthylene productionQuaternary ammonium cationSilicon oxide
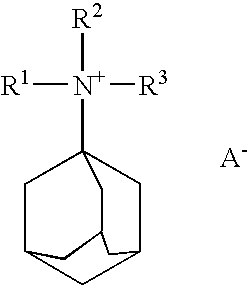
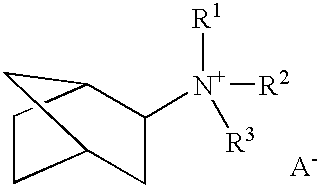
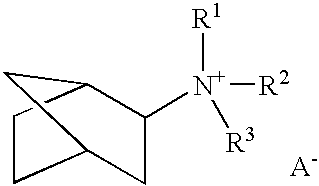
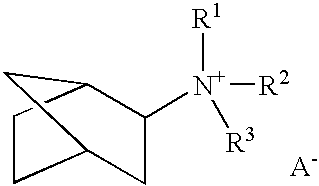
A boron-containing molecular sieve having the CHA crystal structure and comprising (1) silicon oxide and (2) boron oxide or a combination of boron oxide and aluminum oxide, iron oxide, titanium oxide, gallium oxide and mixtures thereof is prepared using a quaternary ammonium cation derived from 1-adamantamine, 3-quinuclidinol or 2-exo-aminonorbornane as structure directing agent. The molecular sieve can be used for gas separation or in catalysts to prepare methylamine or dimethylamine, to convert oxygenates (e.g., methanol) to light olefins, or for the reduction of oxides of nitrogen n a gas stream (e.g., automotive exhaust).
Owner:CHEVROU USA INC
Boron-containing molecular sieve CHA
ActiveUS7226575B2Aluminium compoundsMolecular sieve catalystsQuaternary ammonium cationSilicon oxide
A boron-containing molecular sieve having the CHA crystal structure and comprising (1) silicon oxide and (2) boron oxide or a combination of boron oxide and aluminum oxide, iron oxide, titanium oxide, gallium oxide and mixtures thereof is prepared using a quaternary ammonium cation derived from 1-adamantamine, 3-quinuclidinol or 2-exo-aminonorbornane as structure directing agent. The molecular sieve can be used for gas separation or in catalysts to prepare methylamine or dimethylamine, to convert oxygenates (e.g., methanol) to light olefins, or for the reduction of oxides of nitrogen n a gas stream (e.g., automotive exhaust).
Owner:CHEVROU USA INC
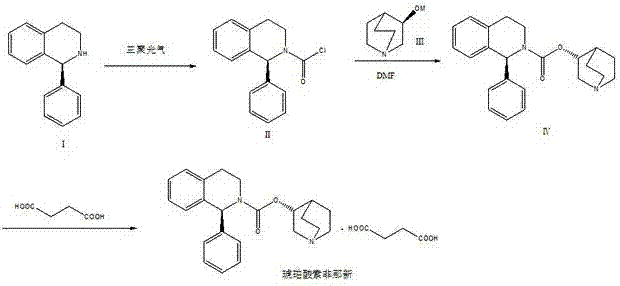
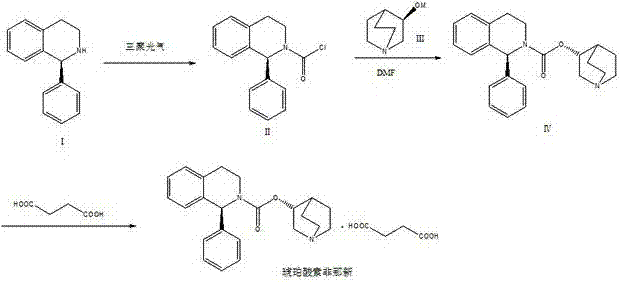
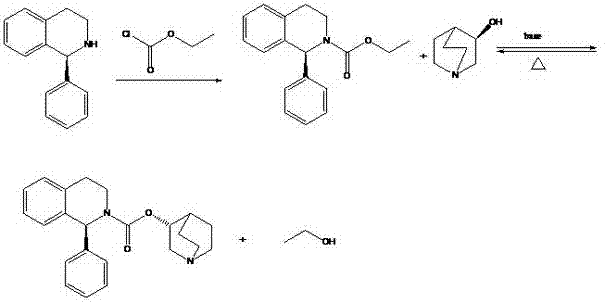
Preparation technology of solifenacin succinate
ActiveCN102875544AImprove conversion rateReverse reaction facilitationOrganic chemistrySolifenacin SuccinateCondensation process
The invention relates to a preparation technology of solifenacin succinate. According to the preparation technology, (S)-1-phenyl-1, 2, 3, 4-tetrahydroisoquinoline carbamyl chloride (II) and (R)-3-quinuclidine alcohol metal salt (III) are reacted to generate solifenacin alkali, and then the solifenacin alkali is prepared into solifenacin succinate. With the adoption of the preparation technology provided by the invention, nucleophilic species which lead to a reverse reaction can be avoided during condensation process, namely, the reverse reaction cannot be generated in the preparation technology, therefore, a transformation rate of the solifenacin succinate is improved, a reaction is greatly promoted, a reaction process is quickly carried out under a mild condition, and the preparation technology is suitable for large-scale production.
Owner:CHENGDU SINO STRONG PHARMA
Process for Production of Optically Active Quinuclidinols
ActiveUS20090216019A1High yieldCheap productionRuthenium organic compoundsGroup 5/15 element organic compoundsRuthenium3-quinuclidinol
A novel ruthenium complex which is a highly efficient catalyst useful for the production of optically active 3-quinuclidinols, and a process for production of optically active 3-quinuclidinols using the ruthenium complex as a catalyst, where the optically active 3-quinuclidinols are useful as an optically active, physiologically active compound utilized in medicines and agrichemicals or as a synthetic intermediate such as a liquid crystal material.
Owner:KANTO CHEM CO INC +1
Methods for producing optically active alcohols
InactiveUS20050227336A1High optical purityEfficient productionSugar derivativesBacteriaTropinone reductase IAlcohol
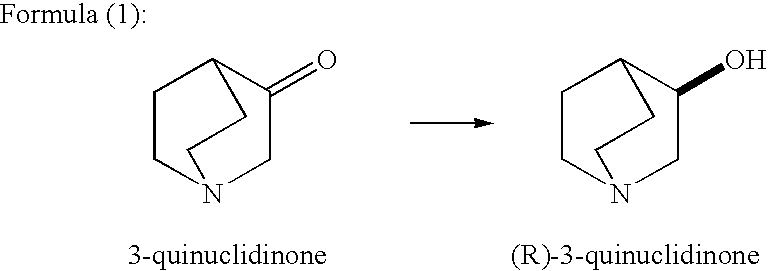
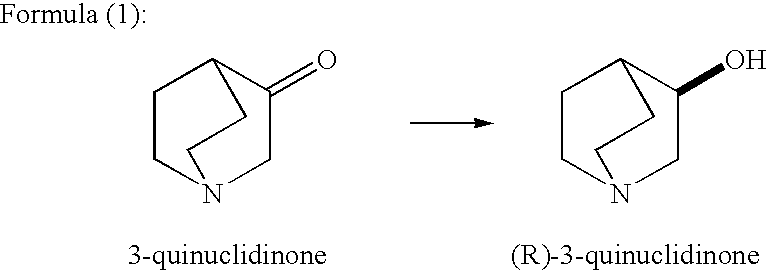
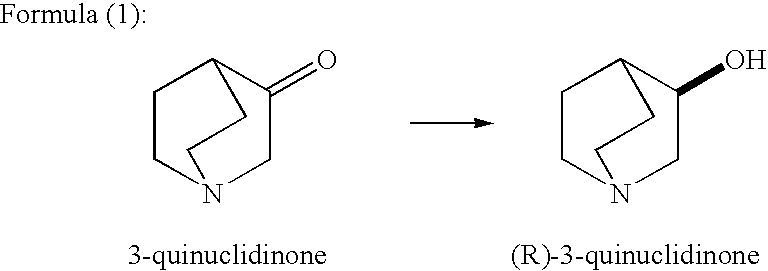
A method for producing optically active alcohols is provided. Optically active alcohols are useful intermediates in pharmaceutical production. The method of the present invention enables simple and efficient production of optically active alcohols with a high optical purity. According to the production method disclosed, optically active alcohols are produced via asymmetric reduction of 3-quinuclidinone using tropinone reductase-I. For example, the use of tropinone reductase-I derived from plants like Datura stramonium and Hyoscyamus niger allows the production of high optical purity (R)-3-quinuclidinol as shown in Formula (1) below.
Owner:DAICEL CHEM IND LTD
Preparation method of impurity in penehyclidine hydrochloride
The invention discloses a preparation method of an impurity in penehyclidine hydrochloride. The preparation method comprises the following steps: taking alpha-phenyl-alpha-cyclopentyl-alpha-hydroxyl ethyl p-toluene sulfonate and 3-quinuclidinol as the raw materials, carrying out reactions for a while under an alkaline condition, performing a post treatment to obtain 3-[2-cyclopentyl-2-phenyl-2-(2-cyclopentyl-2-hydroxyl-2-phenyl-ethyoxyl)ethyoxyl] quinuclidine hydrochloride free alkali, carrying out salt forming reactions between the free alkali and hydrogen chloride, and performing refinement to obtain hydrochloride of the free alkali. The preparation method can prominently increase the impurity content during the preparation process, the operation difficulty is reduced, moreover, the preparation method is suitable for massive production and is capable of obtaining a qualified high purity impurity; and the impurity purity measured by HPLC is 100%.
Owner:海南欣莱医药科技股份有限公司
Imroved alkanolamines for co2 removal from gas streams
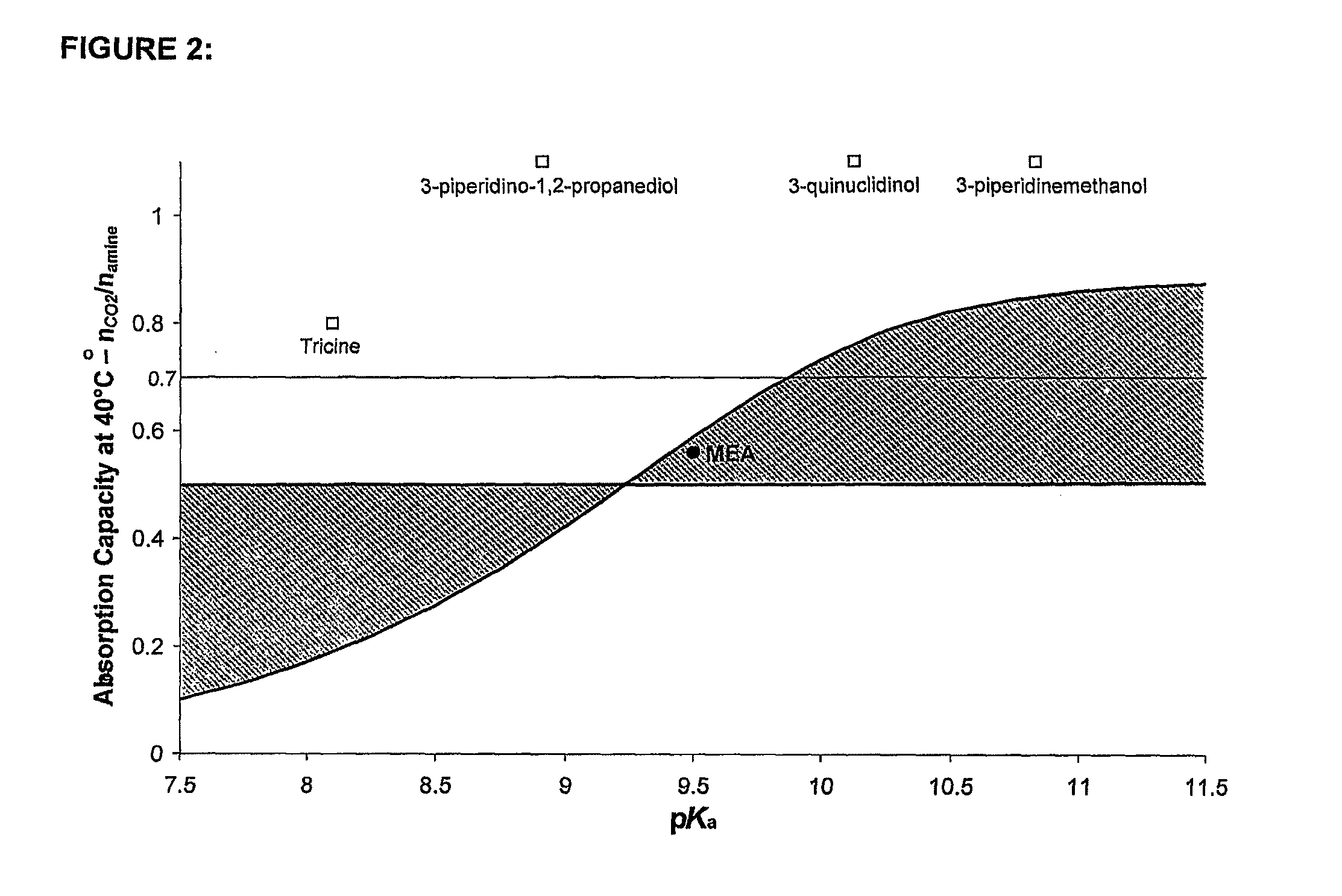
InactiveUS20110116997A1Large CO absorption capacityPoor rateGas treatmentHydrogen sulfidesCo2 removalTricine
A process for the capture of CO2 from gas streams comprising contacting a CO2 containing gas stream with an aqueous alkanolamine solution, wherein the alkanolamine is selected from the group consisting of: 3-piperidinemethanol, Tricine, 3-quinuclidinol, 3-piperidino-1,2-propanediol and their salts.
Owner:COMMONWEALTH SCI & IND RES ORG
Boron-containing molecular sieve cha
InactiveCN101098743AHydrocarbon from carbon oxidesInternal combustion piston enginesQuaternary ammonium cationSilicon oxide
A boron-containing molecular sieve having the CHA crystal structure and comprising (1) silicon oxide and (2) boron oxide or a combination of boron oxide and aluminum oxide, iron oxide, titanium oxide, gallium oxide and mixtures thereof is prepared using a quaternary ammonium cation derived from 1-adamantamine, 3-quinuclidinol or 2-exo-aminonorbornane as structure directing agent. The molecular sieve can be used for gas separation or in catalysts to prepare methylamine or dimethylamine, to convert oxygenates (e.g., methanol) to light olefins, or for the reduction of oxides of nitrogen n a gas stream (e.g., automotive exhaust) and to reduce cold start emissions from engines.
Owner:CHEVROU USA INC
Nocardia sp. capable of converting quininone into (R)-3-quinuclidinol and conversion method
ActiveCN102952761AEasy to trainHigh yieldBacteriaMicroorganism based processes3-quinuclidinolGreen chemistry
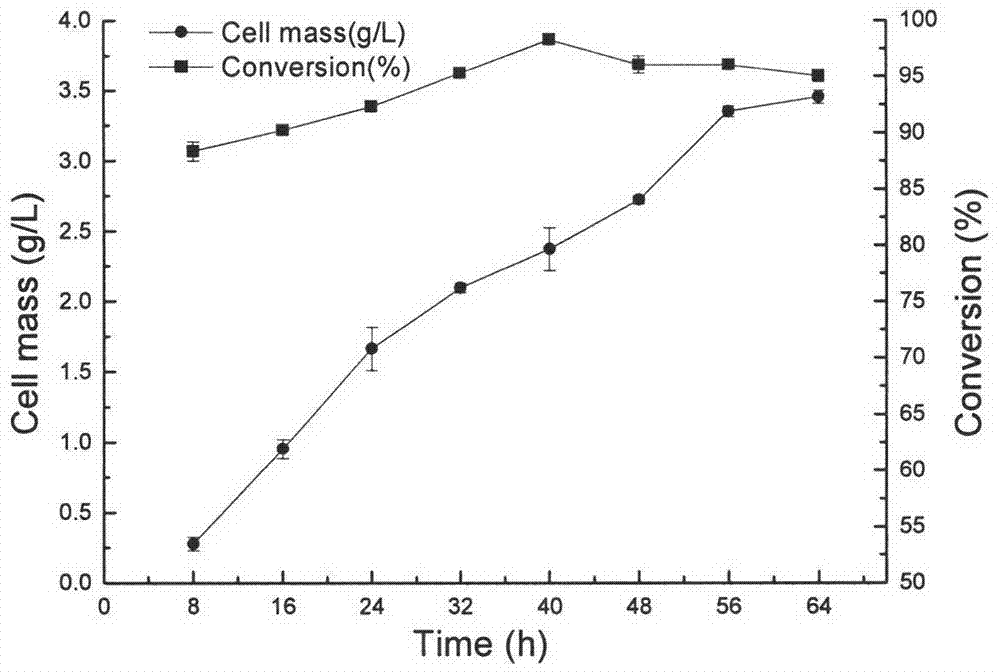
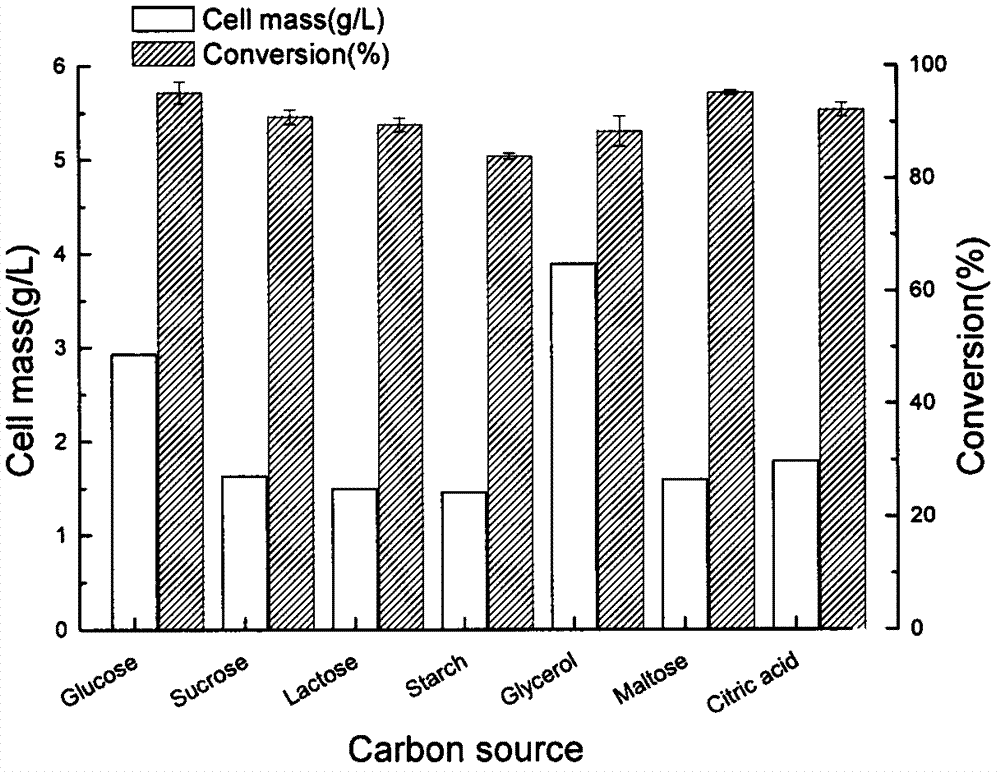
The invention discloses a Nocardia sp. WY1202 and a production method for (R)-3-quinuclidinol through fermentation of quininone by using Nocardia sp. WY1202. According to the invention, quininone hydrochloride is used as a sole carbon source for the strain of Nocardia sp. WY1202 which is screened from soil in a farm orchard in Xiqing District, Tianjin City, China, and is preserved in China General Microbiological Collection Center with an accession number of CGMCC No. 5095. The production method comprises the following steps: with the Nocardia sp.WY1202 as a strain, carrying out primary seed culture and secondary fermentation amplification culture; taking a fermentation culture solution, collecting thalli through centrifugation, washing the thalli and re-suspending the thalli in a buffer solution; adding the substrate quininone hydrochloride, adding glucose and carrying out centrifugation after a reaction for 24 to 48 h; adjusting the pH value of a supernatant until the supernatant is alkaline; drying the supernatant through pressure reduced spinning; subjecting an obtained residue to solid-liquid extraction with an organic solvent; and drying an obtained filtrate through spinning so as to obtain (R)-(-)-3-quinuclidinol. According to the invention, the product (R)-(-)-3-quinuclidinol has a yield of 95%, a purity of 96% and an e.e value of more than 95%; the production method is simple to operate, has low energy consumption, accords with requirements for green chemistry and is applicable to large-scale bioconversion production for (R)-(-)-3-quinuclidinol.
Owner:TIANJIN INST OF IND BIOTECH CHINESE ACADEMY OF SCI
Magnetic combined crosslinked enzyme aggregate biocatalyst and preparation method and application thereof
InactiveCN107227301AIncreased Enzyme LoadingIncrease loadOxidoreductasesFermentationCross-linked enzyme aggregateEnantioselective synthesis
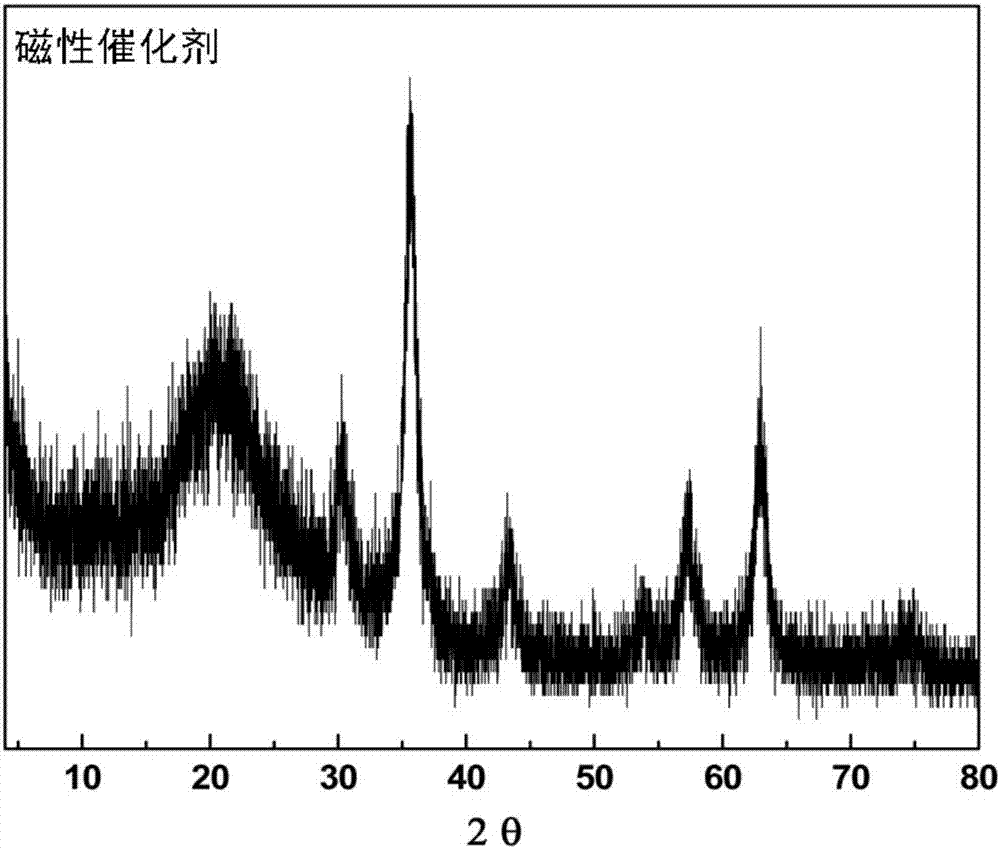
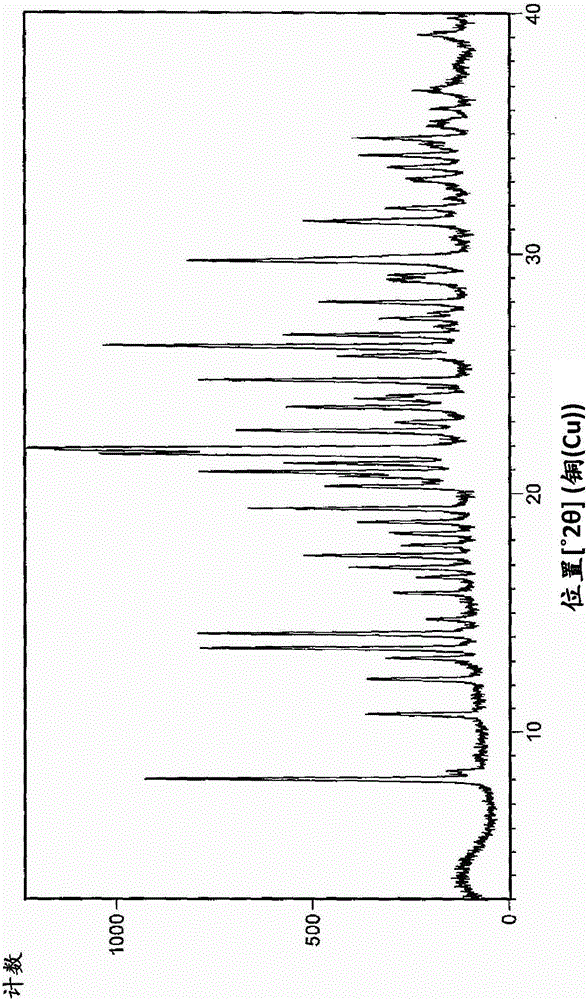
The invention provides a magnetic combined crosslinked enzyme aggregate biocatalyst. The biocatalyst can produce diffraction peaks at diffraction angles 2 theta of 30.5+ / -0.2 degrees, 35.4+ / -0.2 degrees, 44.7+ / -0.2 degrees, 57.3+ / -0.2 degrees and 63.4+ / -0.2 degrees. A preparation method of the biocatalyst is simple, does not need special equipment, needs mild process conditions, is easy to operate, realizes a low cost and is suitable for industrialization. The biocatalyst is used for asymmetric synthesis of (R)-3-quinuclidinol, realizes synchronous carbonyl reduction and coenzyme NADH / NAD<+> in-situ regeneration, prevents diffusional limitation to a substrate , a coenzyme and a product, has good catalytic activity and high catalytic efficiency, greatly shortens reaction time and has obvious economic values.
Owner:CHONGQING MEDICAL UNIVERSITY
Enzyme for the production of optically pure 3-quinuclidinol
The present invention provides a process for production of optically pure quinuclidinol of formula-(I) by reduction of quinuclidinone of formula-(II) in presence of suitable oxidoreductase enzyme derived from Saccharomyces species. Formula (II, I) Moreover, the present enzyme works in presence of cofactor NADP where the cofactor is regenerated by suitable system. The present invention also provides a recombinant vector containing genes co expressing suitable polypeptides having oxido-reductase activity and polypeptide having capacity to regenerate the co-factor. The said vector is transformed in suitable host cell.
Owner:CADILA HEALTHCARE LTD
Treatment of engine exhaust using boron-containing molecular sieve cha
A boron-containing molecular sieve having the CHA crystal structure and comprising (1) silicon oxide and (2) boron oxide or a combination of boron oxide and aluminum oxide, iron oxide, titanium oxide, gallium oxide and mixtures thereof is prepared using a quaternary ammonium cation derived from 1-adamantamine, 3-quinuclidinol or 2-exo-aminonorbornane as structure directing agent.
Owner:YUEN LUN TEH +1
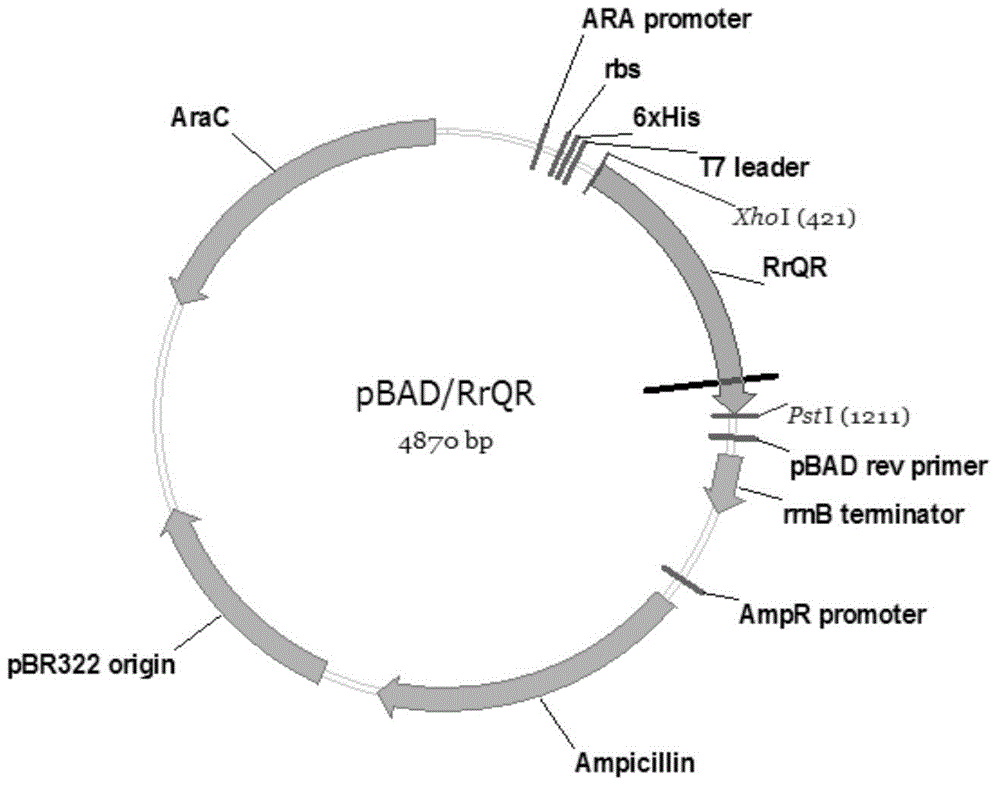
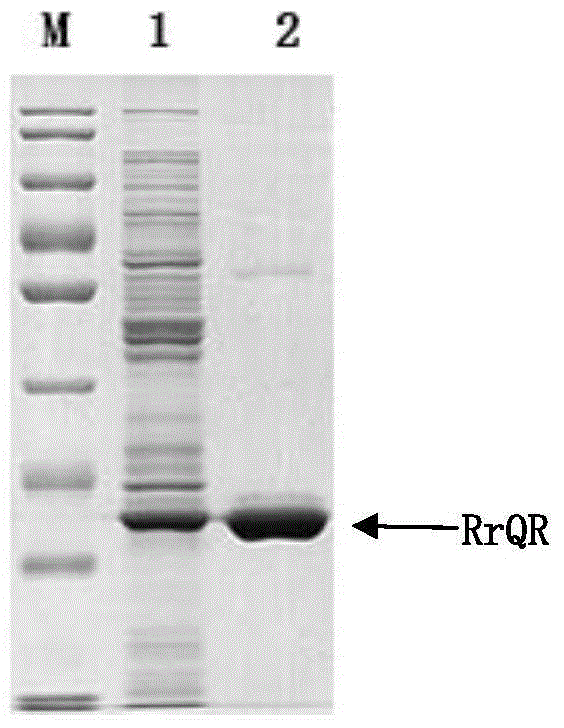
Preparation method of quininone reductase RrQR, and application of quininone reductase RrQR in preparation of (R)-3-quinuclidinol
The invention discloses a preparation method of quininone reductase RrQR, and applications of the quininone reductase RrQR. The applications of the quininone reductase RrQR comprise: an application of the RrQR as quininone reductase; an application of biomaterials related with the RrQR in the preparation of quininone reductase; an application of the RrQR in synthesis of R-3-quinuclidinol; and an application of the biomaterials related with the RrQR in the synthesis of the R-3-quinuclidinol. Experiments prove that the quininone reductase RrQR has high specific vitality and thermal stability and can asymmetrically reduce 3-quininone into optically active R-3-quinuclidinol.
Owner:BIOLOGY INST OF HEBEI ACAD OF SCI +1
Method for preparing (R)-3-quinuclidinol
The invention relates to a method for preparing (R)-3-quinuclidinol, which comprises the following steps: 1)removing salt of 3-quinuclidinol hydrochloride under base interaction to obtain 3-quinuclidone; 2)under anhydrous and anaerobic conditions, by using a chiral catalyst (S,S)xylskewphosrubr2quima and base interaction, and performing asymmetric hydrogenation reduction on 3-quinuclidone to obtain (R)-3-quinuclidinol. The method has beneficial effects that conversion rate of the raw materials can reach more than 99.5%, and ee value of the product can reach more than 95%.
Owner:ENANTIOTECH CORP
An industrially applicable process for preparing high purity aclidinium bromide
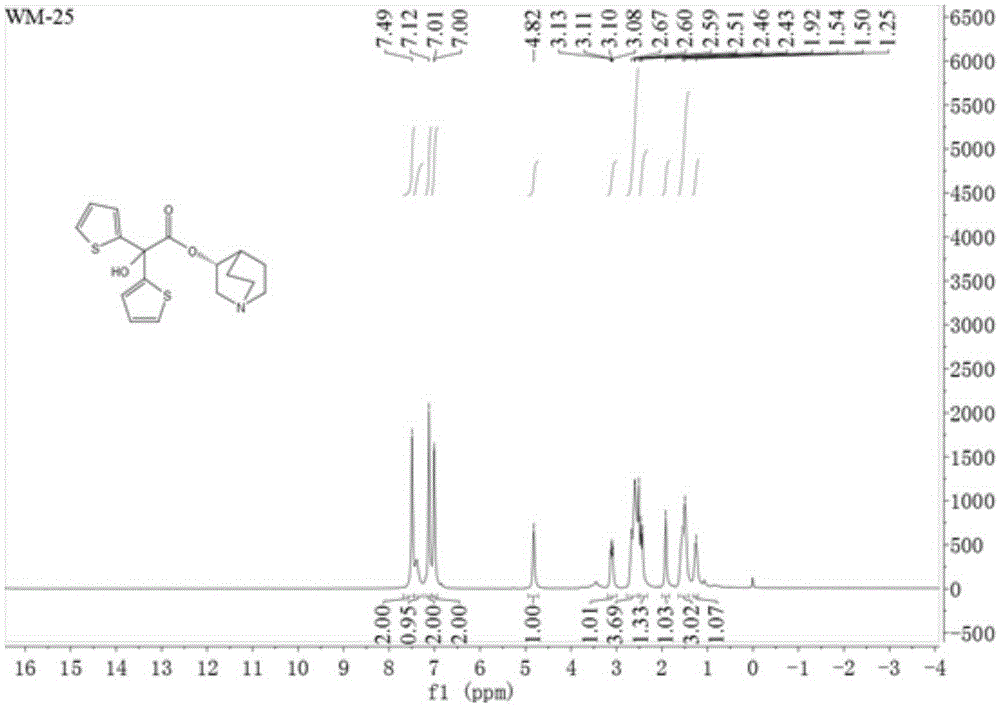
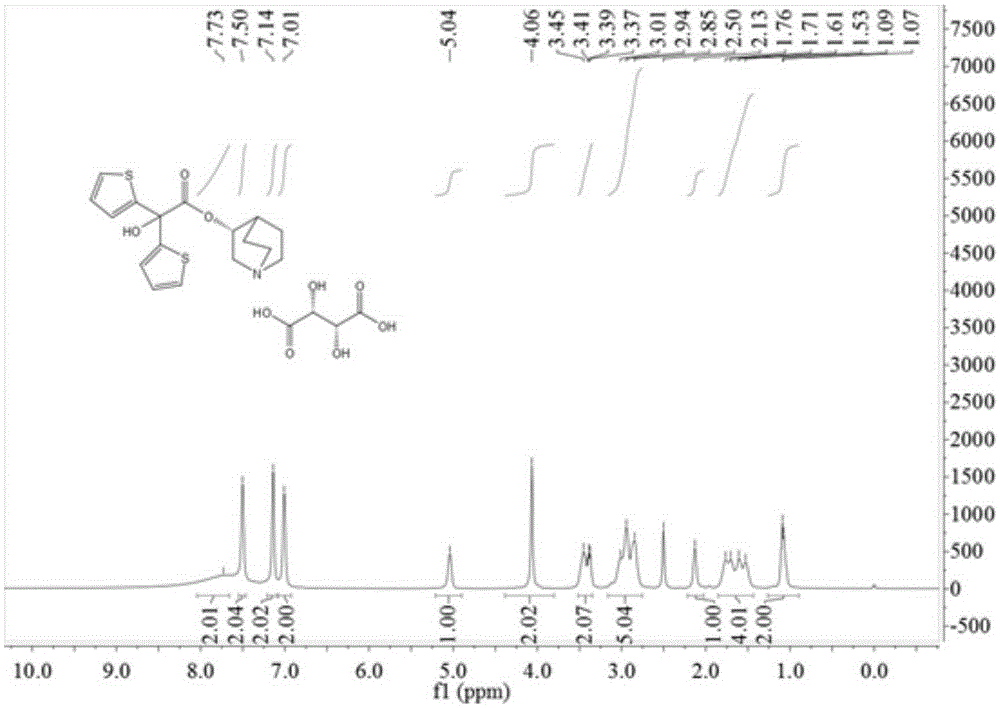
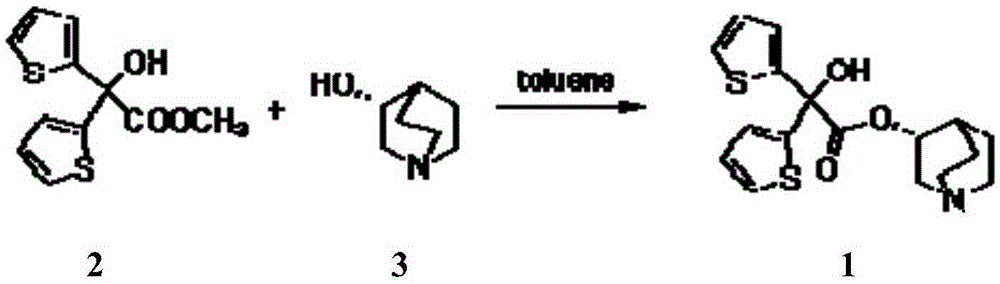
The invention relates to an efficient and industrially applicable process for preparing aclidinium bromide of formula I, comprising the following steps a) preparation of the quinuclidinyl ester by transestenfication of methyl di(2-thienly)giycolate with R-(-)-3-quinuclidinol in the presence of a sterically hindered base in an inert solvent; b) isolation of the quinuclidinyl ester; and c) quaternization of the quinuclidinyl ester by 3-phenoxypropyl bromide in a suitable solvent.
Owner:ZENTIVA AS
Method for preparing (R)-3-quinuclidinol
InactiveCN103980270AHigh enantioselectivityHigh selectivityAsymmetric synthesesAlcohol3-quinuclidinol
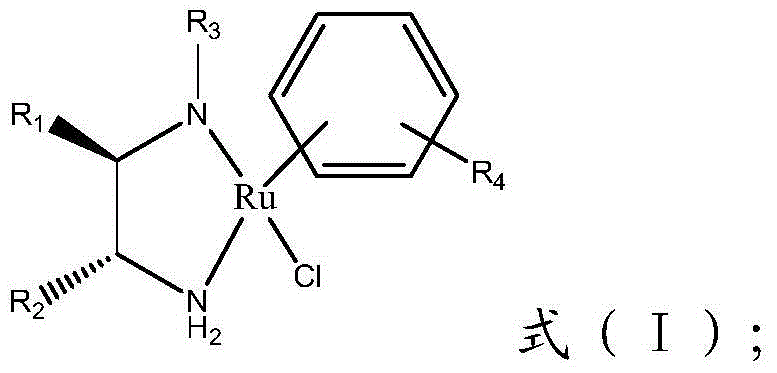
The invention provides a method for preparing (R)-3-quinuclidinol. According to the preparation method, an alcohol reacts with 3-quinuclidone under the action of an alkali and a chiral sulfonamide-ruthenium complex, thus preparing the (R)-3-quinuclidinol. The chiral sulfonamide-ruthenium complex serves as a catalyst, and 3-quinuclidone is subjected to asymmetric transfer hydrogenation synthesis to obtain the (R)-3-quinuclidinol. Compared with the prior art, the method provided by the invention is simple,quixk and efficient in preparation process and has extremely high conversion rate, and the product (R)-3-quinuclidinol with high enantio-selectivity and high yield can be obtained and has excellent industrial application prospect.
Owner:ESTEVE HUAYI PHARMA
Method of detecting impurities in penehyclidine hydrochloride
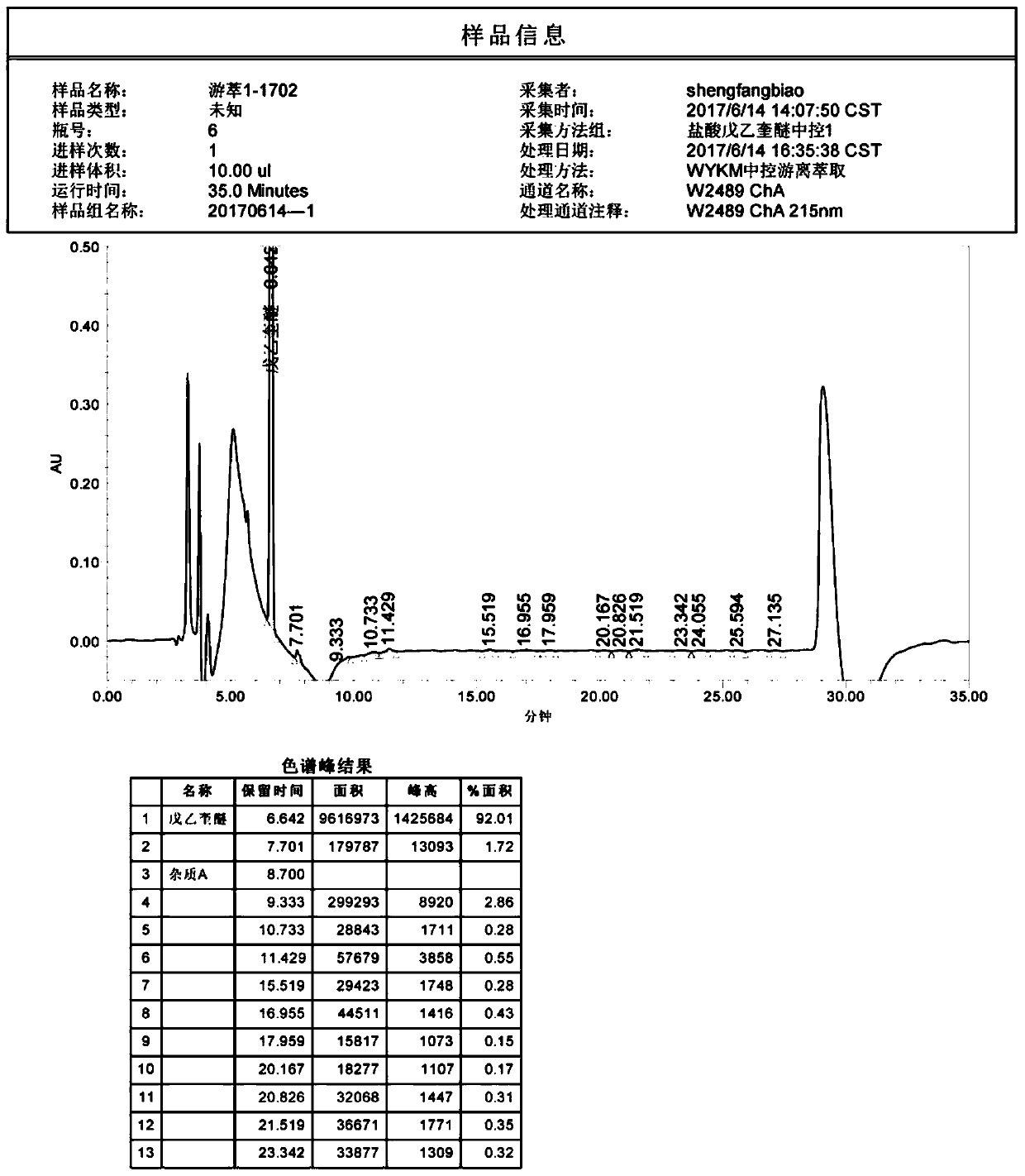
ActiveCN104237393AQuantitative detection of contentImprove controllabilityComponent separationPeak area3-quinuclidinol
The invention discloses a method of detecting two impurities containing a quinuclidine ring in penehyclidine hydrochloride. The method adopts a gas chromatographic method to detect the contents of the impurities, namely the content of 3-quinuclidone and the content of 3-quinuclidinol. During detection, a sample of the penehyclidine hydrochloride is dissolved with an alkaline organic solvent so as to convert all the hydrochlorides into free alkalis and is dried by blowing nitrogen, and then dimethylformamide is added to prepare a sample solution to be detected; and impurity reference substances are processed as the same manner and prepared into impurity reference substance solutions. The sample solution to be detected and the impurity reference substance solutions are directly injected respectively, chromatograms are collected, and the contents of the impurities are calculated based on peak areas by an external standard method. The method of detecting the impurities has characteristics of simple and convenient operation, high sensitivity, capability of quantitative measurement, high accuracy and good reproducibility, effectively controls the product quality of the penehyclidine hydrochloride and guarantees safety and effectiveness of clinical medication.
Owner:重庆科塞亚医药科技有限责任公司
Boron-containing molecular sieve CHA
ActiveUS20060115417A1Aluminium compoundsMolecular sieve catalystsQuaternary ammonium cationSilicon oxide
A boron-containing molecular sieve having the CHA crystal structure and comprising (1) silicon oxide and (2) boron oxide or a combination of boron oxide and aluminum oxide, iron oxide, titanium oxide, gallium oxide and mixtures thereof is prepared using a quaternary ammonium cation derived from 1-adamantamine, 3-quinuclidinol or 2-exo-aminonorbornane as structure directing agent. The molecular sieve can be used for gas separation or in catalysts to prepare methylamine or dimethylamine, to convert oxygenates (e.g., methanol) to light olefins, or for the reduction of oxides of nitrogen n a gas stream (e.g., automotive exhaust).
Owner:CHEVROU USA INC
Method for purifying penehyclidine
Provided is a method for purifying penehyclidine. The method for purifying the penehyclidine comprises the steps of using phenylcyclopentyloxirane and 3-quinuclidinol for synthesizing the penehyclidine, adding water to dilute a penehyclidine reaction solution, then adding methyl tertiary butyl ether, stirring a mixture, standing the mixture for liquid separation, taking an organic phase, adding hydrochloric acid in a concentration of 0.2 mol / L, stirring a mixture, then standing the mixture for liquid separation, adding methyl tertiary butyl ether after taking a water phase, stirring a mixture,adjusting a pH to be 7-8, standing a mixture for liquid separation, and taking a water phase to obtain the purified penehyclidine. Compared with the prior art, the method has the advantages that notonly can more impurities be detected, but also the method has good sensibility of the various impurities. When the method for purifying the penehyclidine is applied specifically, according to detection results of samples in various batches, limits of key impurities are set, so that the economic applicability in a practical production process is greatly improved.
Owner:SHANGHAI XUDONG HAIPU PHARMA
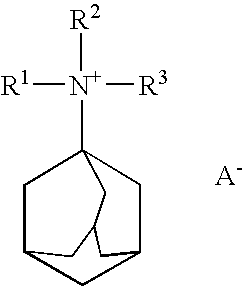
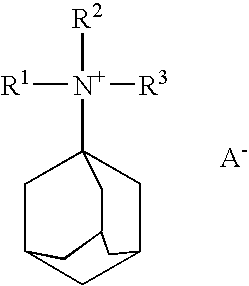
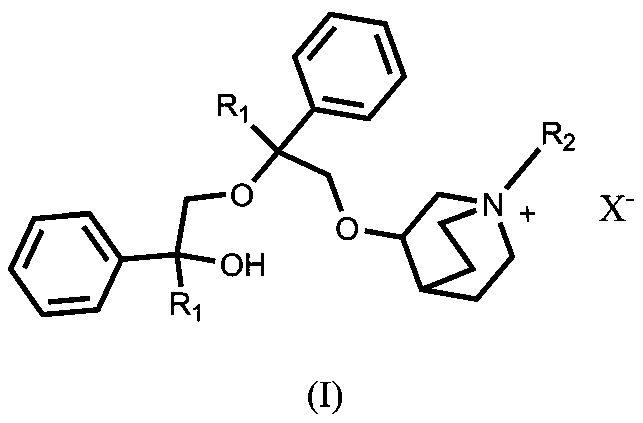
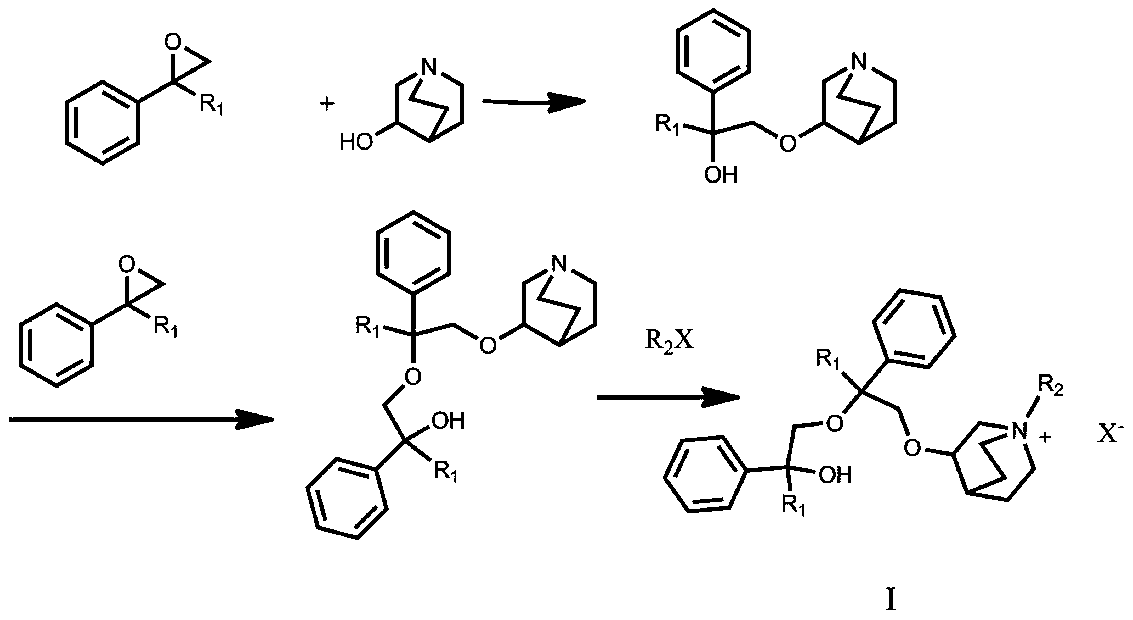
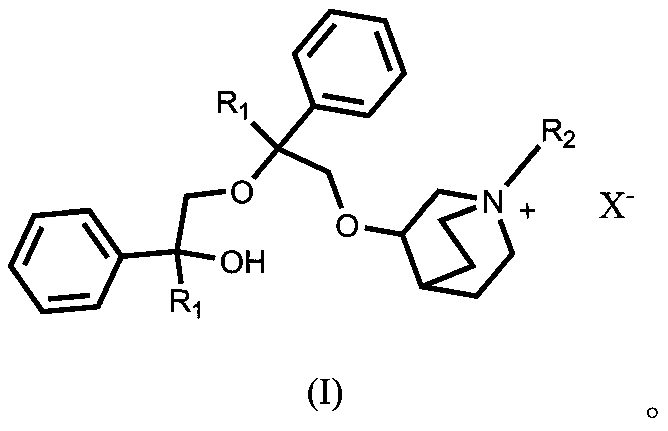
Quinine compound containing quaternary ammonium groups and preparation method of quinine compound
The invention discloses a quinine compound containing quaternary ammonium groups and a preparation method of the quinine compound, and relates to a novel compound with the general formula I and a preparation method of the novel compound. The preparation method comprises the steps that 1-phenyl-1-R1-ethylene oxide and 3-quinuclidinol react in an organic solvent under the action of strong base to obtain 3-(2-R1-2-oxhydryl-2-phenyl ethyoxyl)quinuclidine; the obtained product reacts with 1-phenyl-1-R1-ethylene oxide in the organic solvent under the action of strong base to obtain 3-[2-R1-2-phenyl-2-(2-R1-2-oxhydryl-2-phenyl-ethyoxyl)ethyoxyl] quinuclidine; the obtained product reacts with alkyl halide R2X to obtain (I) (see the formula in the specification).
Owner:山东博洛德生物科技有限公司
Process for producing optically active-3-quinuclidinols
An object of the present invention is to provide a process for producing optically active 3-quinuclidinol having high optical purity or the salt thereof at high yield. The invention relates to a process for producing optically active 3-quinuclidinol or the salt thereof by reacting 3-quinuclidinone or the salt thereof with hydrogen in the presence of a basic compound, a complex of a transition metal in Groups 8 to 10, an optically active bidentate ligand and an optically active diamine.
Owner:TAKASAGO INTERNATIONAL CORPORATION
Process for production of optically active quinuclidinols
ActiveUS8212037B2High yieldCheap productionRuthenium organic compoundsGroup 5/15 element organic compoundsRuthenium3-quinuclidinol
A novel ruthenium complex which is a highly efficient catalyst useful for the production of optically active 3-quinuclidinols, and a process for production of optically active 3-quinuclidinols using the ruthenium complex as a catalyst, where the optically active 3-quinuclidinols are useful as an optically active, physiologically active compound utilized in medicines and agrichemicals or as a synthetic intermediate such as a liquid crystal material.
Owner:KANTO CHEM CO INC +1
R-2,2-di(2-thienyl)-2-glycolic acid quinine-3-ester and preparation and application thereof
ActiveCN105348279AEasy to operateHigh yieldOrganic active ingredientsOrganic chemistryAcetic acidGlycolic acid
The invention relates to R-2,2-di(2-thienyl)-2-glycolic acid quinine-3-ester and preparation and application thereof. A structural formula of the compound is shown in the specification, a reaction is conducted on 2,2-di(2-thienyl)-2-methyl glycolate and 3-quinuclidinol, and 2-hydroxy-2,2-di(2-thienyl) acetic acid-3-quinine ester is obtained; then separation is conducted through chiral acid, alkalization is conducted in an alkali solution, and the R-2,2-di(2-thienyl)-2-glycolic acid quinine-3-ester is obtained. The R-2,2-di(2-thienyl)-2-glycolic acid quinine-3-ester and the preparation and application thereof have the advantages of being simple in reaction operation, high in yield, low in price, short in reaction route, few in three wastes and prone to industrial production.
Owner:DONGHUA UNIV +1
Methods for producing optically active alcohols
InactiveUS7645599B2High optical purityEfficient productionSugar derivativesBacteriaTropinone reductase IAlcohol
A method for producing optically active alcohols is provided. Optically active alcohols are useful intermediates in pharmaceutical production. The method of the present invention enables simple and efficient production of optically active alcohols with a high optical purity. According to the production method disclosed, optically active alcohols are produced via asymmetric reduction of 3-quinuclidinone using tropinone reductase-I. For example, the use of tropinone reductase-I derived from plants like Datura stramonium and Hyoscyamus niger allows the production of high optical purity (R)-3-quinuclidinol.
Owner:DAICEL CHEM IND LTD
Production process of optically active 3-quinuclidinol derivative
InactiveUS8436181B2High optical purityImprove compliance rateBiocideOrganic-compounds/hydrides/coordination-complexes catalystsHalogenHydrogen atom
Owner:NIPPON SODA CO LTD
Synthesis of amines boron-containing molecular sieve CHA
InactiveUS20060115422A1Aluminium compoundsMolecular sieve catalystsQuaternary ammonium cationSilicon oxide
A boron-containing molecular sieve having the CHA crystal structure and comprising (1) silicon oxide and (2) boron oxide or a combination of boron oxide and aluminum oxide, iron oxide, titanium oxide, gallium oxide and mixtures thereof is prepared using a quaternary ammonium cation derived from 1-adamantamine, 3-quinuclidinol or 2-exo-aminonorbornane as structure directing agent. The molecular sieve can be used for gas separation or in catalysts to prepare methylamine or dimethylamine, to convert oxygenates (e.g., methanol) to light olefins, or for the reduction of oxides of nitrogen n a gas stream (e.g., automotive exhaust).
Owner:CHEVROU USA INC
Production process of optically active 3-quinuclidinol derivative
InactiveUS20100174081A1High optical purityImprove compliance rateOrganic-compounds/hydrides/coordination-complexes catalystsCatalytic reactionsHydrogenHydrogen atom
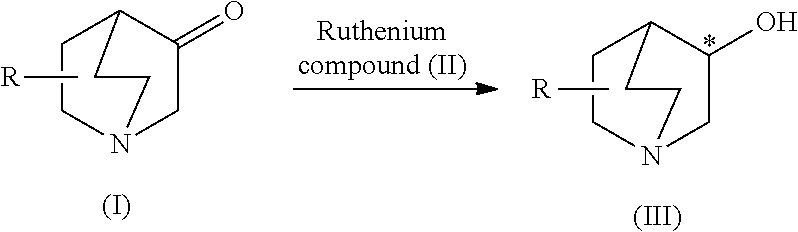
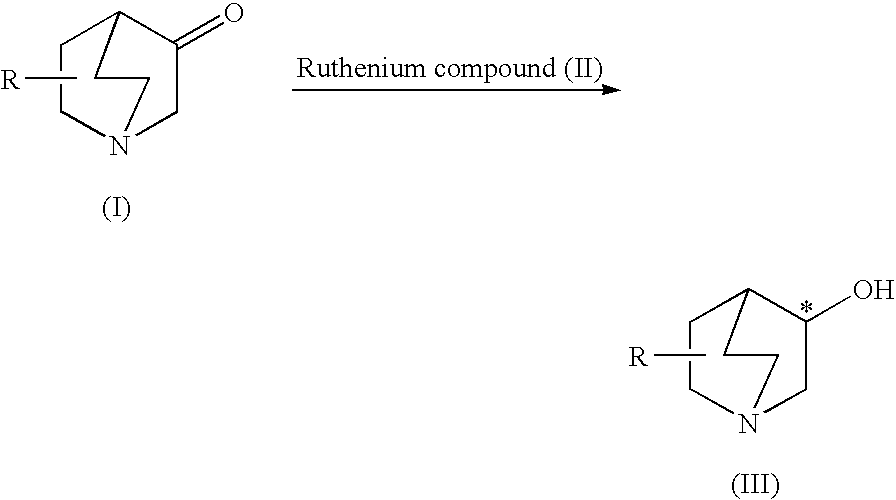
A process is provided for efficiently producing an optically active 3-quinuclidinol derivative of high optical purity using a readily available ruthenium compound as an asymmetric reduction catalyst. This process is a process for producing an optically active 3-quinuclidinol derivative represented by the following formula (III) comprising asymmetrically hydrogenating a 3-quinuclidinone derivative represented by the following formula (I) in the presence of a ruthenium compound (II) represented by formula (II): Ru(X)(Y)(Px)n[R1R2C*(NR3R4)-A-R5R6C*(NR7R8)] (in the formulas, R represents a hydrogen atom or C7 to C18 aralkyl group and the like, X and Y represent hydrogen atoms or halogen atoms and the like, Px represents a phosphine ligand, n represents 1 or 2, R1 to R8 represent hydrogen atoms or C1 to C20 alkyl groups and the like, * represents an optically active carbon atom and A represents an ethylene group and the like).
Owner:NIPPON SODA CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com