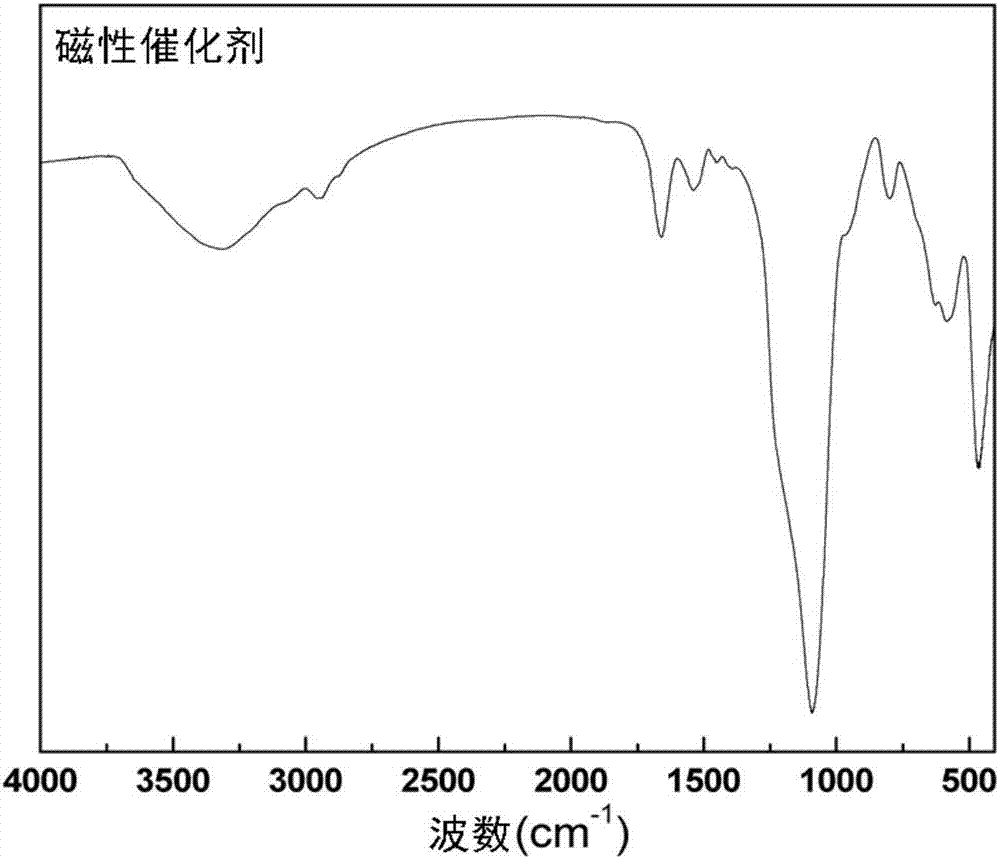
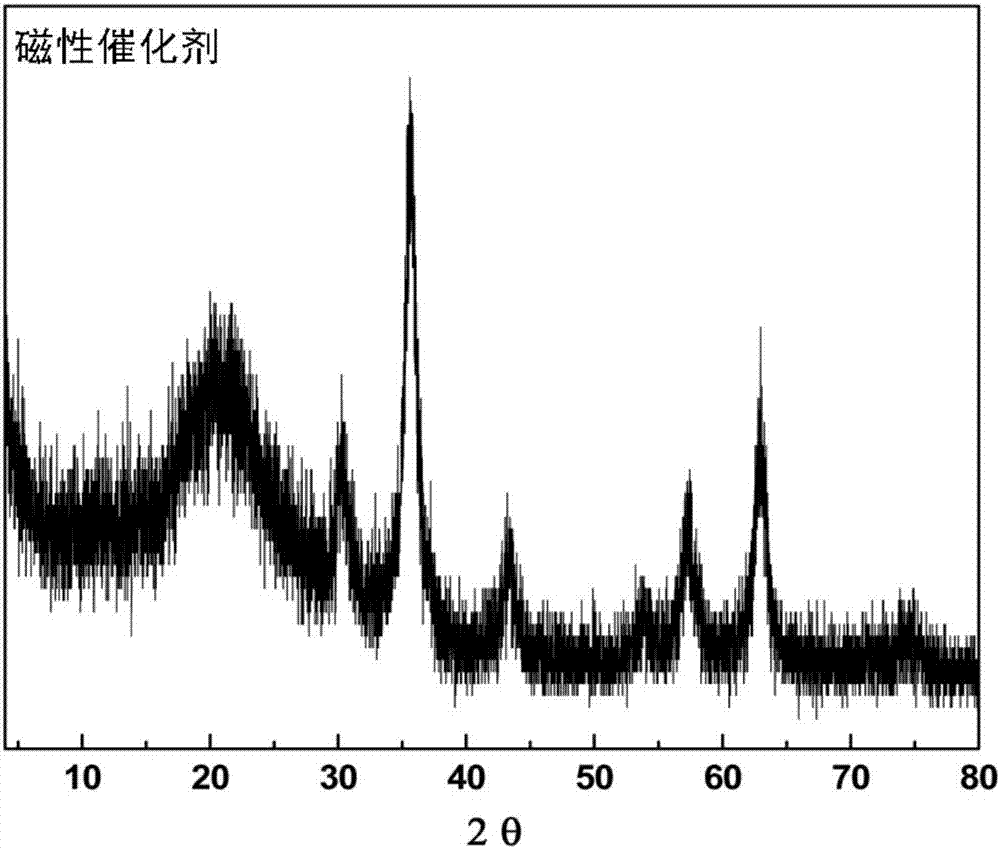
Magnetic combined crosslinked enzyme aggregate biocatalyst and preparation method and application thereof
A biocatalyst and collective biological technology, applied in the field of bioengineering, can solve the problems of easy agglomeration, difficult filtration and recovery, poor mechanical properties, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] QNR enzyme activity assay:
[0056] Standard reaction mixture system: buffer solution B (PBS, pH7.2-7.4), 3 μmol 3-quininone, 0.3 μmol NADH, appropriate amount of enzyme QNR, total volume 1 mL. Changes in absorbance values were measured at λ = 340 nm. Definition of enzyme activity unit: the amount of enzyme required to convert 1 μmol NADH within 1 min at 25°C.
[0057] GDH enzyme activity assay:
[0058]Standard reaction mixture system: buffer solution B (PBS, pH7.2-7.4), 10 μmol glucose, 1 μmol NAD + , an appropriate amount of enzyme GDH, in a total volume of 1 mL. Changes in absorbance values were measured at λ = 340 nm. Enzyme activity unit definition: 1 μmol NAD converted within 1 min at 25°C + The amount of enzyme required.
Embodiment 2
[0060] Magnetic Fe 3 o 4 Preparation of nanoparticles:
[0061] FeCl with a mass ratio of 2:1 3 ·6H 2 O (10.8116g) and FeCl 2 4H 2 O (3.9762g) was dissolved in 100-200mL deionized water, under nitrogen protection, and mechanically stirred at a speed of 500-1500r / min for 0.5-1.0 hours. Add 10-30mL NaOH solution with a concentration of 8-12M dropwise, and mechanically stir at a speed of 1000-2000r / min for 1-1.5 hours. Raise the temperature to 70-100°C, mature for 0.5-2.0 hours; cool down to room temperature, magnetically separate and wash with deionized water for 3-5 times, dry to obtain magnetic Fe 3 o 4 nanoparticles;
[0062] Magnetic Fe 3 o 4 Amino-functionalization of nanoparticles:
[0063] Magnetic Fe 3 o 4 100-200 mg of nanoparticles, dispersed in 140-280 mL of ethanol / water solution with a volume ratio of 5:2, under nitrogen protection, stirred at 20-80 ° C, 400-1000 r / min for 1.0-3.0 hours. Add 2-4mL concentrated ammonia water, stir for 0.5-1.5 hours, add...
Embodiment 3
[0065] Joint cross-linking immobilization of QNR and GDH:
[0066] Get the amino-functionalized magnetic Fe prepared in Example 2 3 o 4 Nanoparticles 5mg, dispersed in 1.0mL phosphate buffered saline solution (PBS, 10mM, pH7.2-7.5) containing QNR (4.0mg / mL) and GDH (2.0mg / mL), at 4°C, speed 600r / min Stir for 0.5 hours. Add 9 volumes of ice-cold precipitant (saturated ammonium sulfate), and stir for 1.5 hours. Add 1.0 mL of glutaraldehyde with a concentration of 40 mM, and stir at 400 r / min for 8.0 h. Magnetic separation, washed 3 times with PBS, to obtain magnetic combi-CLEAs.
[0067] The magnetic combi-CLEAs prepared in embodiment 3 is carried out scanning electron microscope analysis
[0068] Drop the sample solution onto a clean cover glass, dry it in vacuum at 40°C, spray gold to cover the sample, and image it with a scanning electron microscope (SEM, S-3000N type). The analysis results are shown in the attached figure 1 . attached figure 1 It is combi-CLEAs micro...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com