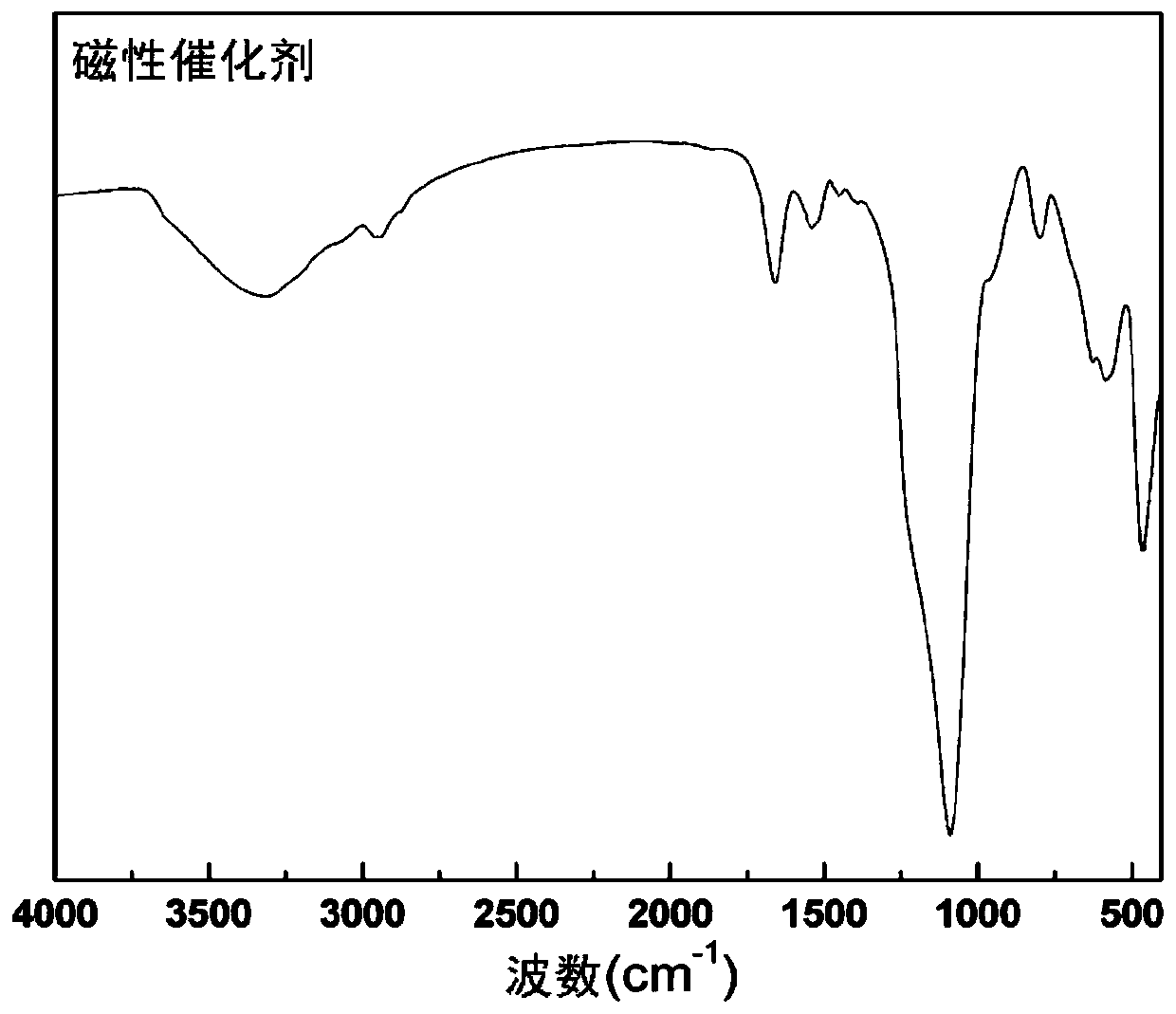
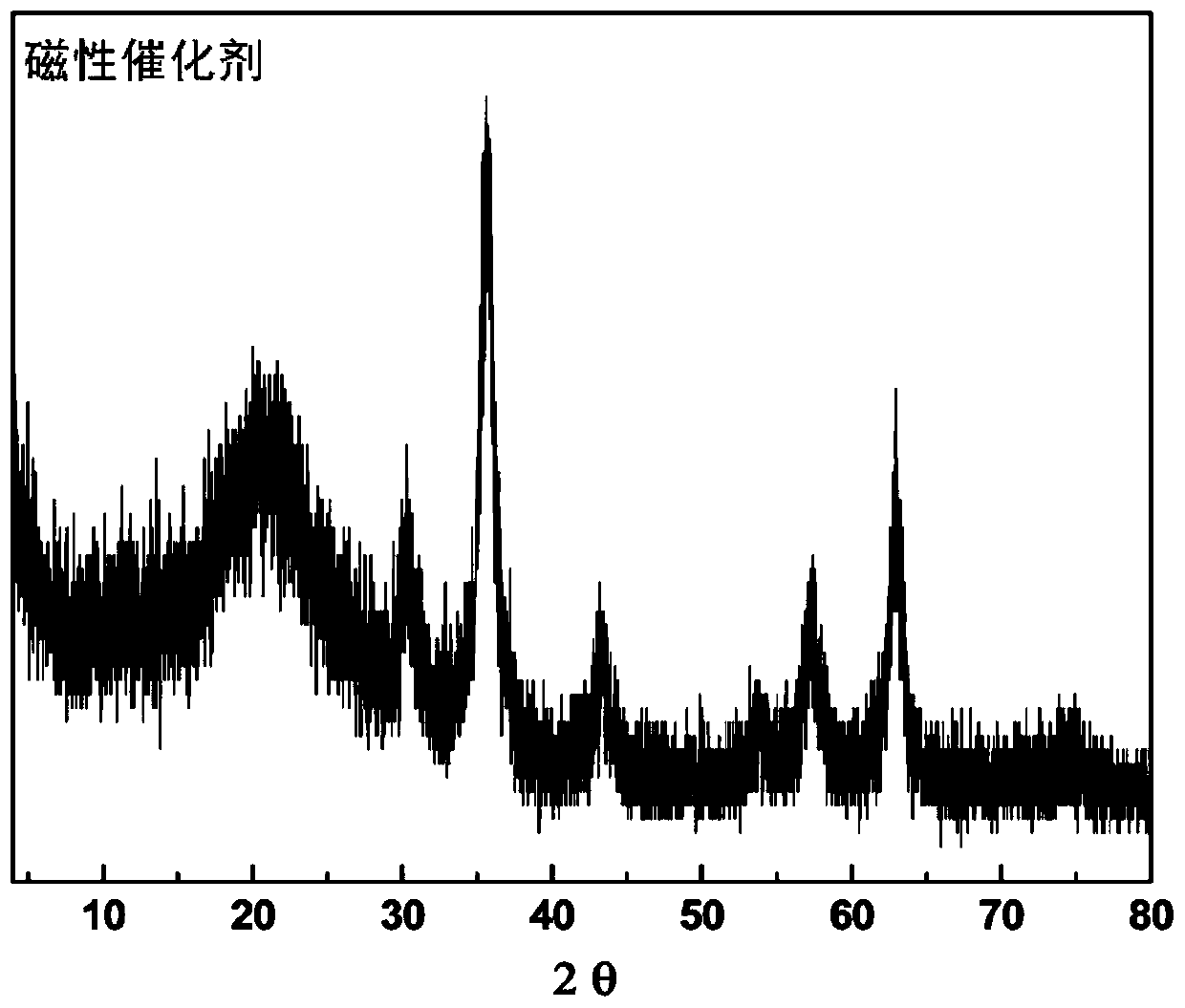
Magnetic combined cross-linked enzyme aggregate biocatalyst and preparation method and application thereof
A biocatalyst and collective biotechnology, applied in the field of bioengineering, can solve problems such as difficult filtration and recovery, easy aggregation into blocks, and obstacles to the industrial application of CLEAs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] QNR enzyme activity assay:
[0056] Standard reaction mixture system: buffer solution B (PBS, pH7.2-7.4), 3 μmol 3-quininone, 0.3 μmol NADH, appropriate amount of enzyme QNR, total volume 1 mL. Changes in absorbance values were measured at λ = 340 nm. Definition of enzyme activity unit: the amount of enzyme required to convert 1 μmol NADH within 1 min at 25°C.
[0057] GDH enzyme activity assay:
[0058]Standard reaction mixture system: buffer solution B (PBS, pH7.2-7.4), 10 μmol glucose, 1 μmol NAD + , an appropriate amount of enzyme GDH, in a total volume of 1 mL. Changes in absorbance values were measured at λ = 340 nm. Enzyme activity unit definition: 1 μmol NAD converted within 1 min at 25°C + The amount of enzyme required.
Embodiment 2
[0060] Magnetic Fe 3 o 4 Preparation of nanoparticles:
[0061] FeCl with a mass ratio of 2:1 3 ·6H 2 O (10.8116g) and FeCl 2 4H 2 O (3.9762g) was dissolved in 100-200mL deionized water, under nitrogen protection, and mechanically stirred at a speed of 500-1500r / min for 0.5-1.0 hours. Add 10-30mL NaOH solution with a concentration of 8-12M dropwise, and mechanically stir at a speed of 1000-2000r / min for 1-1.5 hours. Raise the temperature to 70-100°C, mature for 0.5-2.0 hours; cool down to room temperature, magnetically separate and wash with deionized water for 3-5 times, dry to obtain magnetic Fe 3 o 4 nanoparticles;
[0062] Magnetic Fe 3 o 4 Amino-functionalization of nanoparticles:
[0063] Magnetic Fe 3 o 4 100-200 mg of nanoparticles, dispersed in 140-280 mL of ethanol / water solution with a volume ratio of 5:2, under nitrogen protection, stirred at 20-80 ° C, 400-1000 r / min for 1.0-3.0 hours. Add 2-4mL concentrated ammonia water, stir for 0.5-1.5 hours, add...
Embodiment 3
[0065] Joint cross-linking immobilization of QNR and GDH:
[0066] Get the amino-functionalized magnetic Fe prepared in Example 2 3 o 4 Nanoparticles 5mg, dispersed in 1.0mL phosphate buffered saline solution (PBS, 10mM, pH7.2-7.5) containing QNR (4.0mg / mL) and GDH (2.0mg / mL), at 4°C, speed 600r / min Stir for 0.5 hours. Add 9 volumes of ice-cold precipitant (saturated ammonium sulfate), and stir for 1.5 hours. Add 1.0 mL of glutaraldehyde with a concentration of 40 mM, and stir at 400 r / min for 8.0 h. Magnetic separation, washed 3 times with PBS, to obtain magnetic combi-CLEAs.
[0067] The magnetic combi-CLEAs prepared in embodiment 3 is carried out scanning electron microscope analysis
[0068] Drop the sample solution onto a clean cover glass, dry it in vacuum at 40°C, spray gold to cover the sample, and image it with a scanning electron microscope (SEM, S-3000N type). The analysis results are shown in the attached figure 1 . attached figure 1 It is combi-CLEAs micro...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com