Method for preparing (R)-3-quinuclidinol
A technology of quinine alcohol and quinine ketone, which is applied in the field of preparation of -3-quinine alcohol, and can solve the problems of long-term cumbersome post-treatment process, low yield and efficiency, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
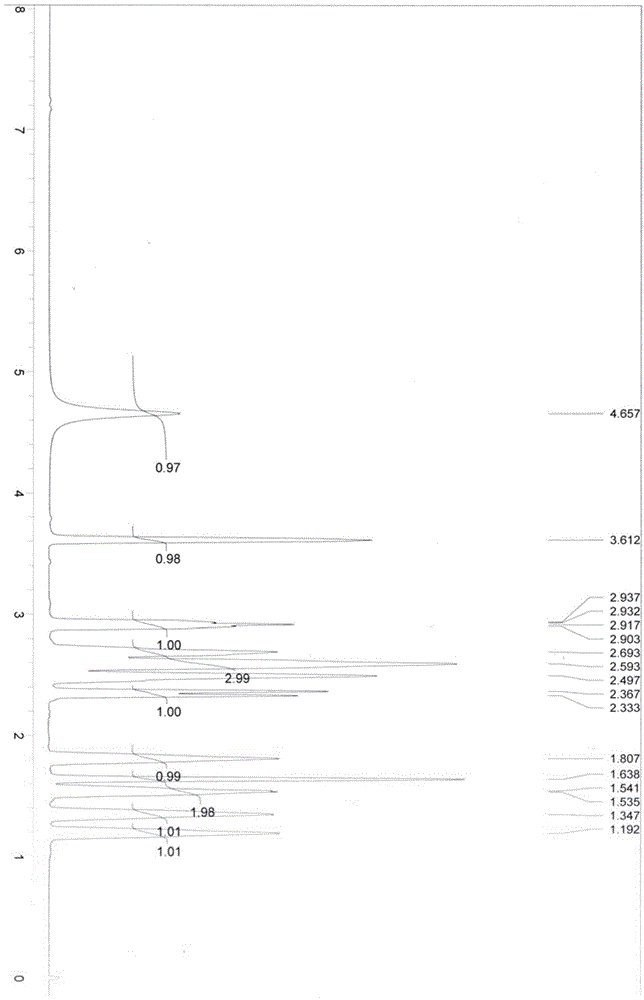
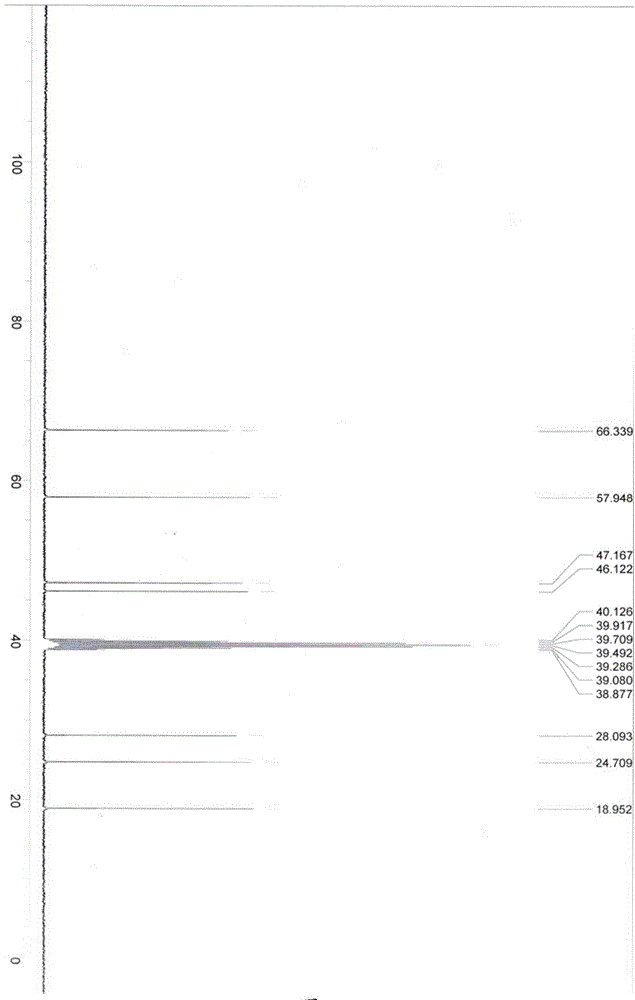
Image
Examples
Embodiment 1
[0063] Add 3-quininone hydrochloride (3.23g, 20mmol), potassium hydroxide (1.40g, 25mmol), water (300mL) into a 500mL separatory funnel, then add 50mL of dichloromethane, shake well and let stand to separate layers , release the dichloromethane layer of the lower floor, and extract the dichloromethane solution to obtain 3-quininone; repeat the extraction step of the above dichloromethane twice, and combine the organic layers, and then use the dichloromethane solution obtained with anhydrous After drying over magnesium sulfate, filtering, and concentrating under reduced pressure, the solvent was removed to obtain a white solid (2.00 g, 16 mmol), which was 3-quininone, which was sealed and protected from light until use.
Embodiment 2
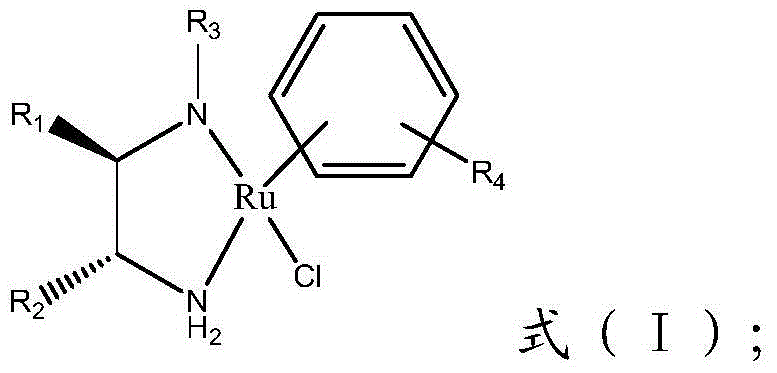
[0065] Add (1R,2R)-cyclohexanediamine (0.571g, 5mmol) and dichloromethane (125mL) into a 150mL round bottom flask, cool to 0°C in an ice-salt bath, then slowly add N dropwise to the reaction system, N-Dimethylsulfonyl chloride (0.718g, 5mmol), after the dropwise addition, the system was slowly warmed up to room temperature, stirred overnight, and then the resulting mixture was distilled under reduced pressure to obtain a viscous liquid, which was purified by a silica gel column (The eluent is dichloromethane / isopropanol (v:v=6:1)) to obtain a white solid (536mg, 2.42mmol), which is the chiral ligand represented by formula (II-2).
Embodiment 3
[0067] Add (1R,2R)-cyclohexanediamine (2.28g, 20mmol) and dichloromethane (125mL) into a 150mL round-bottomed flask, cool to 0°C in an ice-salt bath, and then slowly dropwise add Toluenesulfonyl chloride (1.14g, 6mmol) in dichloromethane (30mL) solution, after the dropwise addition, the system was slowly warmed up to room temperature, stirred overnight, and then the resulting mixed solution was distilled under reduced pressure to obtain a viscous liquid, Purify it with a silica gel column (eluent is dichloromethane / methanol (v:v=10:1)) to obtain a light red solid (680 mg, 2.53 mmol), which is a chiral compound represented by formula (II-3). Ligand.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com