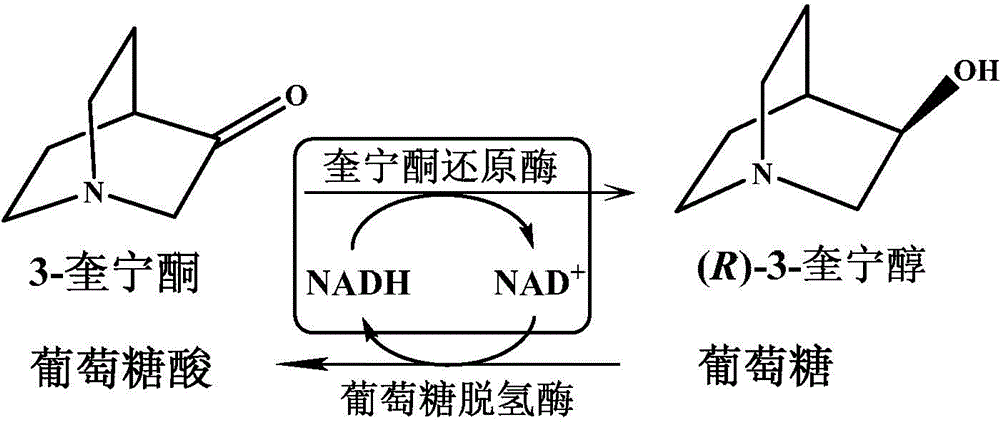
Quininone reductase and application thereof to asymmetric synthesis of (R)-3-quinuclidinol
A technology of quinine ketone and reductase, which is applied in the field of quinine reductase and its application in the asymmetric synthesis of (R)-3-quinine alcohol, can solve the problem of unsuitable for industrial production, low product concentration, The low activity of the enzyme itself has achieved good industrial application prospects, high product concentration, and easy operation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach
[0038] The present inventor carried out the functional measurement of quinine reduction from the wild bacteria preserved in this laboratory, and selected the strains capable of reducing 3-quinine to generate (R)-3-quinine alcohol, obtained by the shotgun method. The asymmetric catalytic reduction of 3-quinine ketone to generate (R)-3-quinine alcohol gene, thus completing the present invention.
[0039] The present invention is further illustrated below by means of examples, but the present invention is not limited thereto. For the experimental methods that do not specify specific conditions in the following examples, select according to conventional methods and conditions, or according to the product instructions.
[0040] The sources of material in the following examples are:
[0041] Agrobacterium radiobacter CGMCC7986.
[0042] The expression plasmid pET28a was purchased from Shanghai Novagen Company.
[0043] E.coli DH5α and E.coli BL21(DE3) competent cells, 2×Taq PCR M...
Embodiment 1
[0045] Embodiment 1 Wild-type Agrobacterium radiata catalyzes the asymmetric reduction of 3-quininone
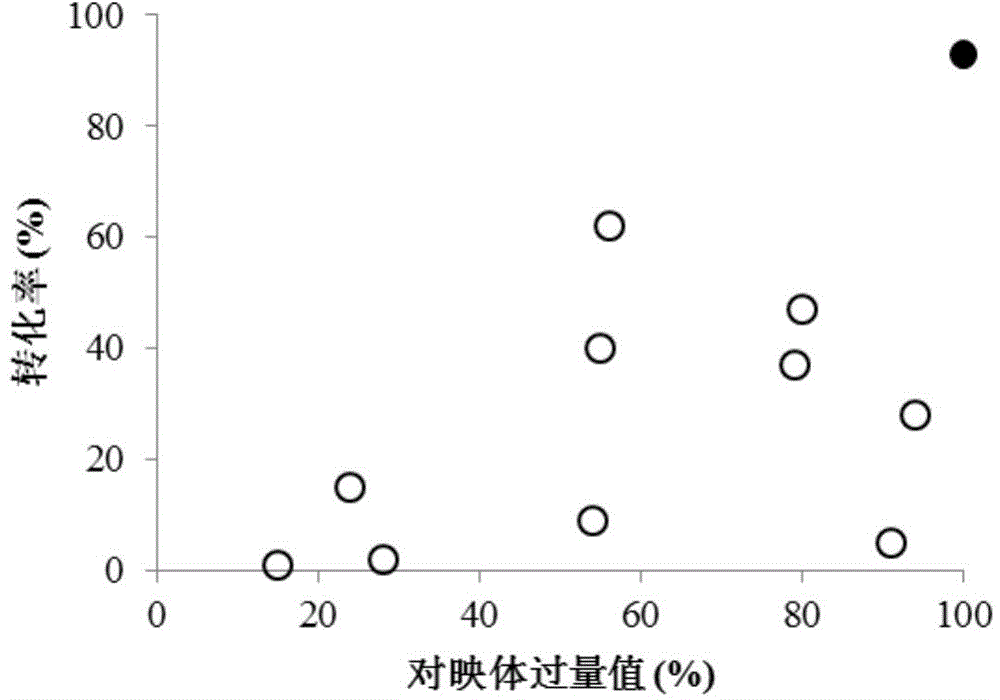
[0046] Agrobacterium radiobacter (Agrobacterium radiobacter) CGMCC7986 was cultured at 30°C for 2 days in a medium containing peptone 5g / L, meat extract 3g / L, pH 7.0. Gained culture fluid is centrifuged to obtain thalline precipitation, and the wet cell of Agrobacterium radiatus described in 10g / L is used as a biocatalyst, and when the substrate concentration is 10mM, the conversion rate of quinine can be made to reach 93% within 12 hours , the optical purity of the product is 99%ee(R).
[0047] Cloning of embodiment 2 quinine reductase gene
[0048] Based on the open reading frame obtained by shotgun cloning, the PCR primers were designed as follows:
[0049] Upstream primer: GAATTC CATATG GAGGCTTCATTGTCGG;
[0050] The downstream primer is: CGC GGATCC TCAGTCCATGCGAACGCCAC
[0051] Wherein, the underlined part in the upstream primer is the NdeI restriction site, and...
Embodiment 3
[0053] Embodiment 3 Preparation of recombinant expression plasmid and recombinant expression transformant
[0054] The PCR product containing the quinine reductase gene obtained in Example 2 was double-digested with restriction endonucleases NdeI and BamHI at 37°C for 12 hours, purified by agarose gel electrophoresis, and purified using an agarose gel DNA recovery reagent box to recover the target fragment. Under the action of T4 DNA ligase, the target fragment was ligated with the vector plasmid pET28a digested with NdeI and BamHI at 4°C overnight to obtain the recombinant expression plasmid pET-ArQR.
[0055] Transform the above-mentioned recombinant expression plasmid into Escherichia coli (E.coli) DH5α competent cells, screen the positive recombinants on the resistance plate containing kanamycin, pick a single clone, and the colony PCR verification is positive clone. Cultivate the recombinant bacteria, extract the plasmid after the plasmid is amplified, retransform into ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com