Nocardia sp. capable of converting quininone into (R)-3-quinuclidinol and conversion method
A Nocardia and quinine alcohol technology, applied in the field of bioengineering, can solve the problems of 3-quinine alcohol with low optical purity and derivative reactions, and achieve strong adaptability to the catalytic environment, simple reaction process, and catalytic reaction The effect of mild conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] Embodiment 1: Screening and identification of strains
[0051] 1.1 Medium
[0052] Inorganic salt medium: K 2 HPO 4 ·3H 2 O 1.0g, Na 2 HPO 4 ·3H 2 O 1.0g, (NH 4 ) 2 HPO 4 2.0g, NaNO 3 2.0g, MgSO 4 ·7H 2 O 0.2g, CaCl 2 2H 2 O 10mg, FeSO 4 ·7H 2 O 1.0mg, ZnSO 4 0.1mg, distilled water 1000mL.
[0053] A certain concentration of quinine hydrochloride was added to the inorganic salt medium to prepare the corresponding enrichment medium.
[0054] 1.2 Isolation and purification of strains
[0055] Put 5g of the collected sample into a 250mL shaker flask containing 50mL of sterile water, add glass beads, shake on a shaker at 250r / min for 2h, and let it stand for 3-5min. After the soil particles settle, take 2mL of the supernatant and add Into 50mL liquid enrichment medium (containing quinine hydrochloride 5g / L). After 3-4 days of culture at 30° C. on a shaker at 200 r / min, transfer to the next batch of enrichment medium (containing quinine hydrochloride 7...
Embodiment 2
[0059] Example 2: Catalytic properties of Nocardia sp.WY1202
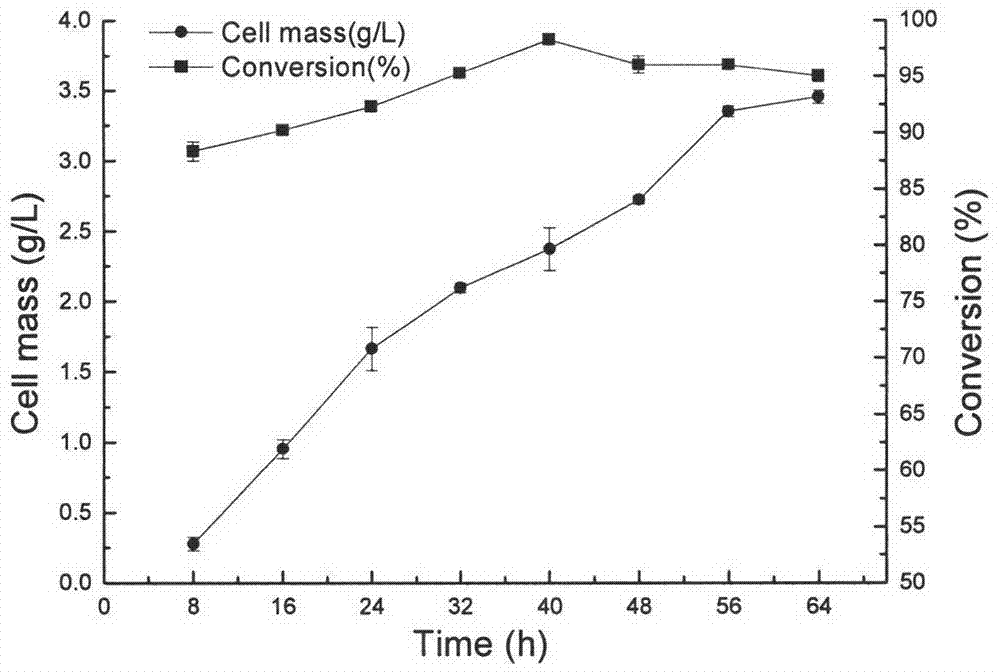
[0060] The growth curve and enzyme production curve of Nocardia sp.WY1202 cultured at 30°C in the fermentation medium in embodiment 1 are as follows: figure 2 shown. Depend on figure 2 It can be seen that in the first 40h of bacterial cell culture, the conversion rate increases with the growth of the bacterial cell, and the conversion rate is the highest in the middle and late logarithmic growth phase (40h), reaching 92.7%. In the later stage of cell culture, the conversion rate of quinine ketone began to decline, but the decline was not obvious.
Embodiment 3
[0061] Embodiment 3: Preliminary optimization of Nocardia sp.WY1202 fermentation conditions
[0062] 1 Single factor experiment of carbon source
[0063] The addition amount of different carbon sources in the fermentation medium was 15g / L.
[0064] Table 1, the reaction of different carbon source fermentation bacteria transforming quinine into (R)-3-quinine alcohol
[0065]
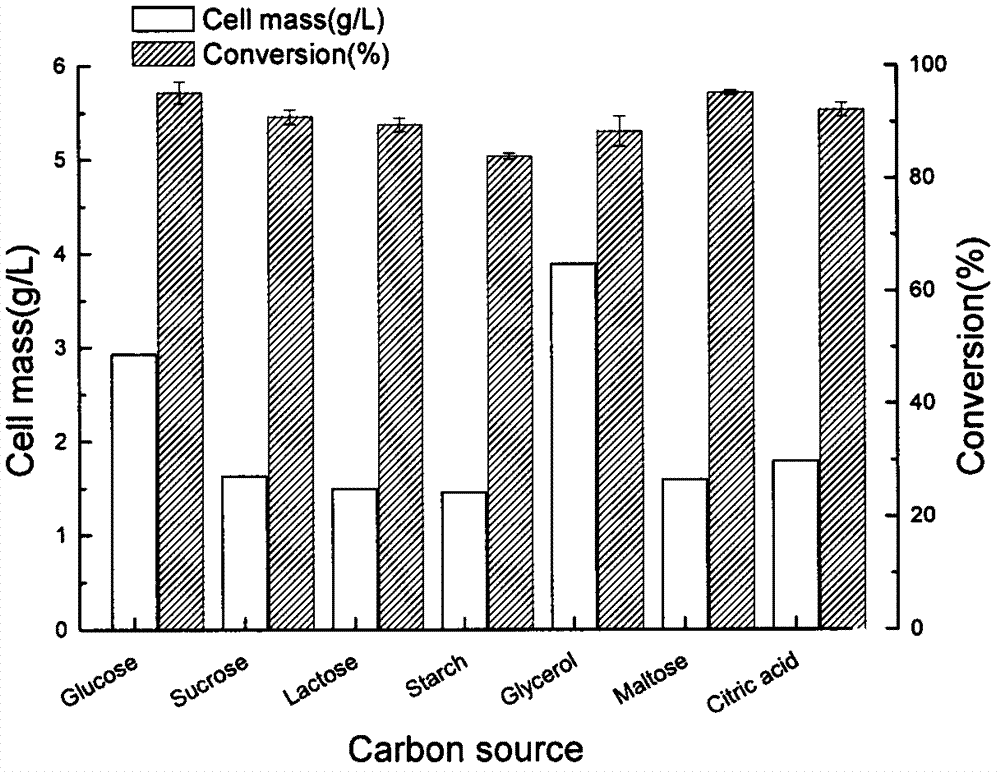
[0066] from image 3 It can be seen from the results that glycerol is the most suitable carbon source for cell growth, followed by glucose, but for the conversion of quinine, glycerol is not a particularly ideal carbon source. When glucose was used as the carbon source, the conversion rate of quinine ketone was the highest. Considering the conversion rate and dry cell weight comprehensively, glucose was the optimal carbon source.
[0067] 2 Single factor experiment of nitrogen source
[0068] The addition amount of different nitrogen sources in the fermentation medium was 10g / L.
[0069] Table 2, ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| boiling point | aaaaa | aaaaa |
| specific rotation | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com