Compound and pharmaceutical composition for treating nasal oversecreation and chronic obstructive pulmonary disease
A compound and COPD technology, applied in the field of pharmaceutical compounds, can solve the problems of short half-life and achieve the effect of long half-life and no side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] Embodiment one: the preparation of compound
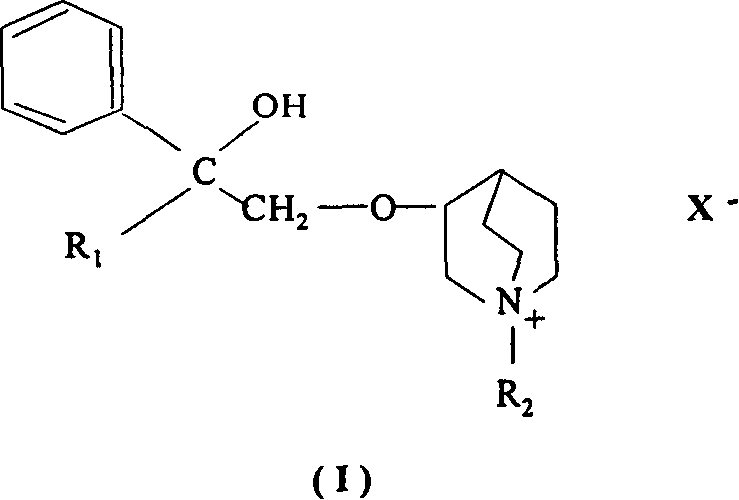
[0049] With 3-{(2-cyclopentyl-2-hydroxyl-2-phenyl)ethoxy}-1-methyl-bromide-1-azabicyclo{2,2,2,}octane (compound α, its cost has been detailed in Chinese invention patent 03121031.7) as an example:
[0050] 1. Preparation of intermediate product: 9.3 g of 1-phenyl-1-cyclopentyl oxirane was dissolved in DMSO. Take 6.35g of quinine alcohol, dissolve it in 64ml of DMSO, add 2.5g of sodium hydride, stir for 1 hour, cool to room temperature, add dropwise phenylcyclopentyl oxirane-DMSO solution, and stir for another 3 hours. Cool to room temperature, extract with ether, extract the ether layer with 6N HCl, alkalinize the acidic aqueous layer with 20% NaOH, extract with ether, and dry over anhydrous sodium sulfate. The solvent was evaporated, and the product was purified by distillation to obtain the intermediate product 3-quinuclidinyl-(2'-phenyl-2'-cyclopentyl-2'-hydroxy)ethyl ether (yield 54%);
[0051] 2. Preparation of compo...
Embodiment 2
[0063] Embodiment two: composition and its preparation
[0064] Preparation and dosage of nasal drops, nasal sprays, aerosols, and powder sprays.
[0065] 1. Unilateral nasal drops
[0066] The composition of nasal drops consists of the following elements
[0067] Compound V
12mg
3mg
h 2 o
10ml
[0068] Under 100,000-level production conditions, the active ingredient (compound V) and bacteriostatic agent (benzalkonium chloride) were dissolved in H 2 In O, it is filled in a small brown bottle with a spindle-shaped cap, which can be used for nasal drops.
[0069] The unilateral nasal drop can dissolve 2.0-25mg of the compound V in every 10ml of water, and can be formulated into nasal drops with different concentrations;
[0070] Single nasal drops 2-3 drops (0.1ml-0.15ml) / nostril each time, about 20-300μg / nostril dosage.
[0071] 2. Compound nasal drops
[0072] Compound II
2.5mg
Fen...
Embodiment 3
[0098] Embodiment 3: the detection and the mensuration of biological half-life after dog nasal spray administration and mouse intramuscular injection administration
[0099] 1. Nasal spray for dogs
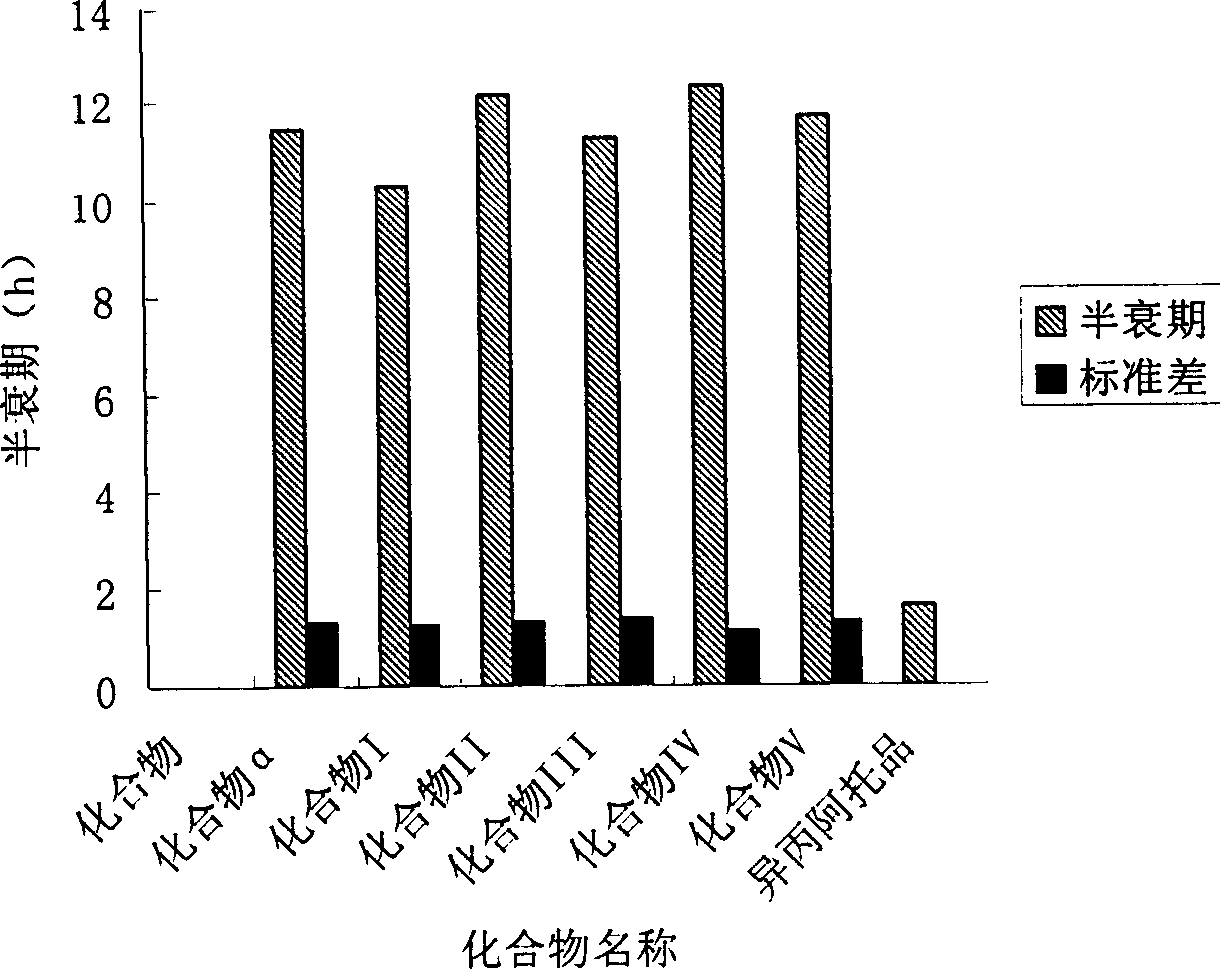
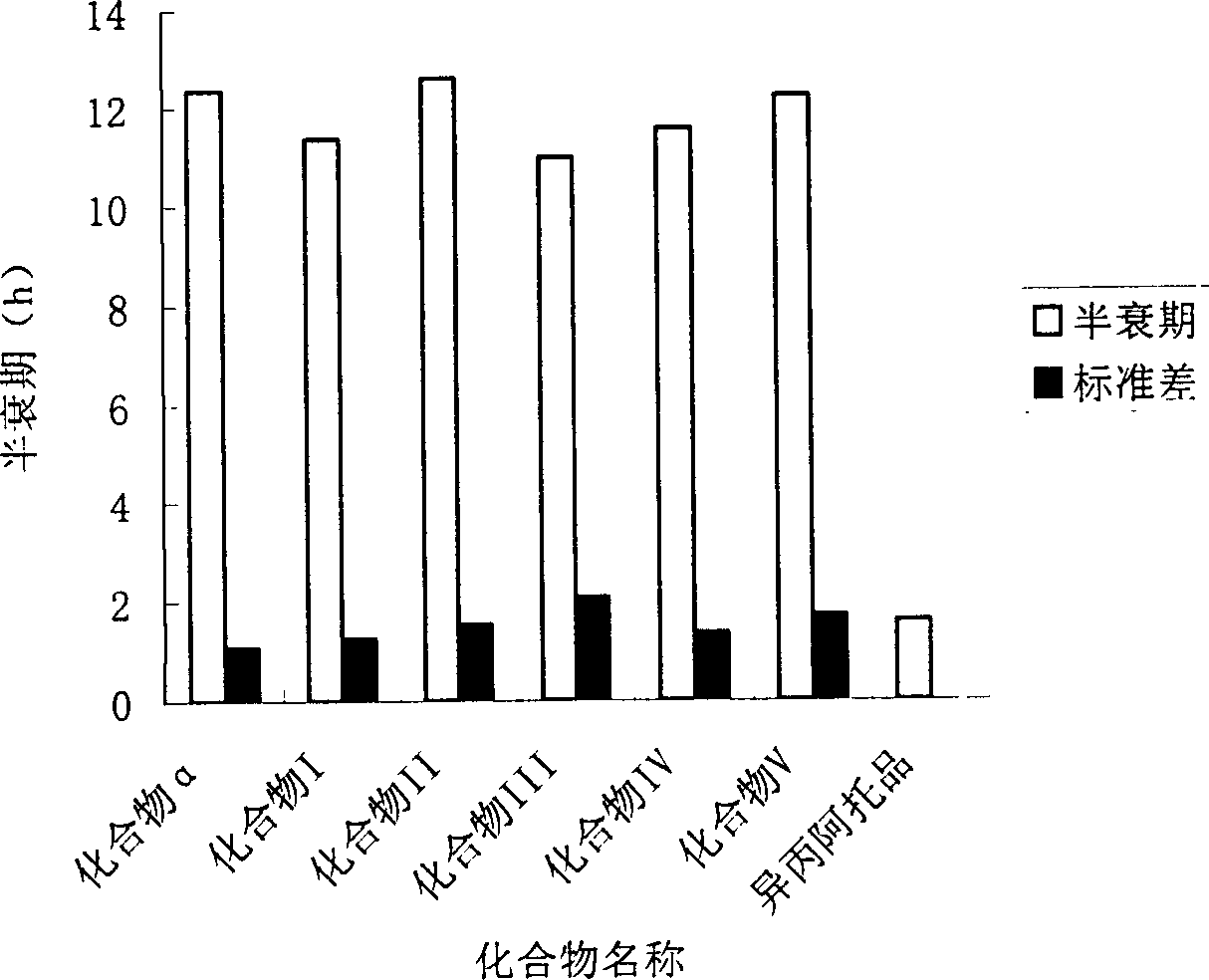
[0100] Beagle dogs were used in the experiment, and each 1 mg of compound α, I, II, III, IV, and V was given nasal spray for pharmacokinetic test, and the blood drug concentration was determined by GC-MS / SIM analysis method. Analyze the drug-time curve in blood and calculate the corresponding pharmacokinetic parameters. The results show that the absorption rates of the six compounds after nasal spray are 19.8±1.2%, 20.5±3.2%, 22.1±3.3%, and 20.7±4.3%. , 19.5±2.7%, 21.6±3.4%, 15min after administration, the given compound can be detected in all dog blood, 30min blood drug concentration reaches a higher level, 30-40min reaches a peak. After 2 hours, the blood drug concentration decreased slowly, and the blood drug concentration maintained a considerable concentration for at least 4...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com