A kind of detection method of impurity in penhyclidine hydrochloride determined by high performance liquid chromatography
A penehyclidine hydrochloride, detection method technology, applied in the direction of measuring devices, instruments, scientific instruments, etc., can solve the problems of inability to meet the requirements of impurity detection, insignificant color development, large operation errors, etc., and achieves high accuracy and sensitivity. High, the effect of improving the detection sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] The selection of embodiment 1 wavelength
[0043] Take the appropriate amount of impurity A, impurity B, and penhyclidine hydrochloride respectively, dissolve them in methanol, and perform ultraviolet full-wavelength scanning in the wavelength range of 190-400nm. The results show that: penehyclidine hydrochloride, impurity A, and impurity B There is a large absorption in the range of ~220nm. Among them, impurity A has maximum absorption at 208nm, impurity B has maximum absorption at 209nm, and penhyclidine hydrochloride has maximum absorption at 208nm. Since the cut-off wavelength of acetonitrile is 190nm, in order to ensure the separation and detection of impurities as much as possible and to avoid the interference of low-band, 210nm±10nm was selected as the detection wavelength.
Embodiment 2
[0044] The mensuration of embodiment 2 sample
[0045] Main chromatographic conditions:
[0046] Mobile phase: mobile phase A: acetonitrile; mobile phase B: 0.02mol / L potassium dihydrogen phosphate (containing 0.5% triethylamine, dilute phosphoric acid to adjust pH=3.0). Carry out gradient elution according to the table below:
[0047]
[0048] Flow rate: 1.0ml / min.
[0049] Column temperature: 35°C.
[0050] Detection wavelength: 210nm.
[0051] Injection volume: 20μl
[0052] Preparation of the test solution: Accurately weigh 10 mg of penhyclidine hydrochloride for the test, add solvent (mobile phase A: mobile phase B=32:68) to dissolve and set the volume to 10 ml, and obtain the test solution (1 mg / ml).
[0053]Preparation of the reference substance solution: Accurately weigh 10 mg of the impurity reference substance, add solvent (mobile phase A: mobile phase B=32:68) to dissolve and set the volume to 100ml, precisely pipette 1ml, add solvent to dissolve and set t...
Embodiment 3
[0056] Embodiment 3 Methodological verification test
[0057] According to the chromatographic conditions of Example 2, methodological verification was carried out from aspects of specificity, precision, linearity, detection limit and quantification limit, stability, and the results are as follows:
[0058] (1) Specificity:
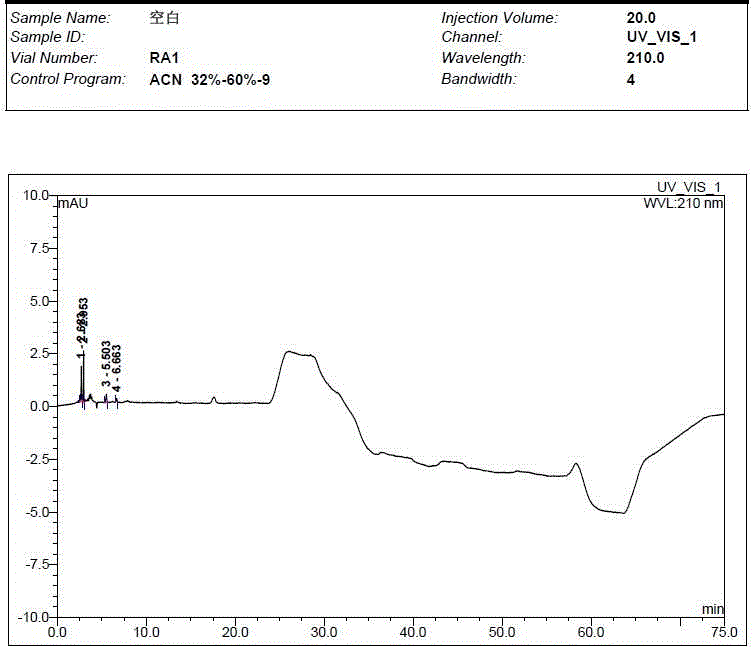
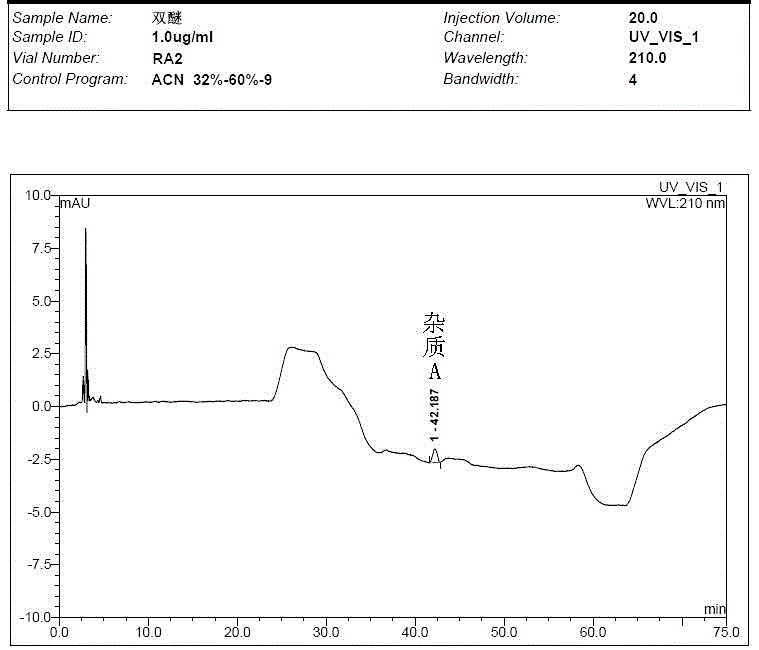
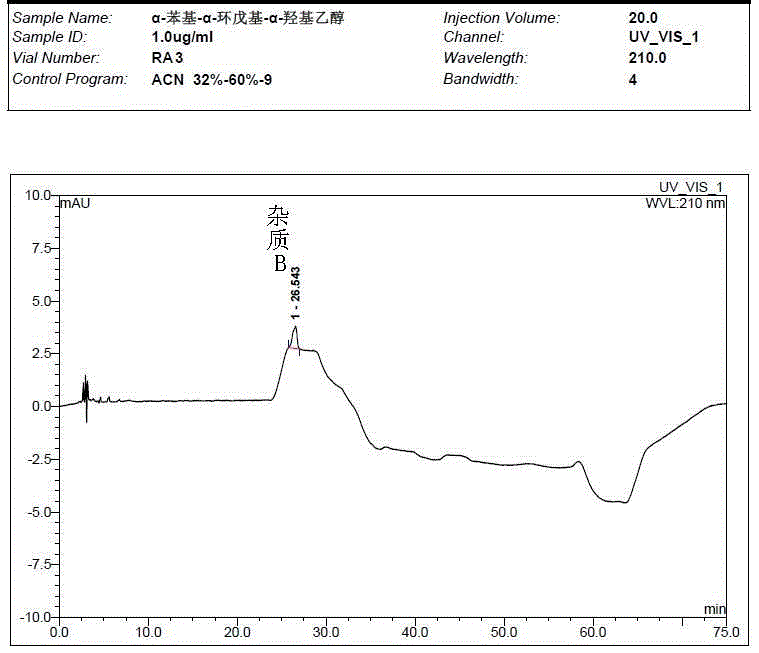
[0059] Take the solvent, the test solution (pentehyclidine hydrochloride solution), the mixed solution of impurity A and B reference substance, the mixed solution of penehyclidine hydrochloride and impurity reference substance A and B, and inject samples in sequence. The test results showed that the peak eluting time of the solvent was between 2min and 5min, the retention time of penhyclidine hydrochloride was 11.633min and 12.420min, the retention time of impurity A was 42.035min, and the retention time of impurity B was 25.577min. The results showed that the separation between the solvent and the reference substance solution was good, and there was no ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| correlation coefficient | aaaaa | aaaaa |
| correlation coefficient | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com