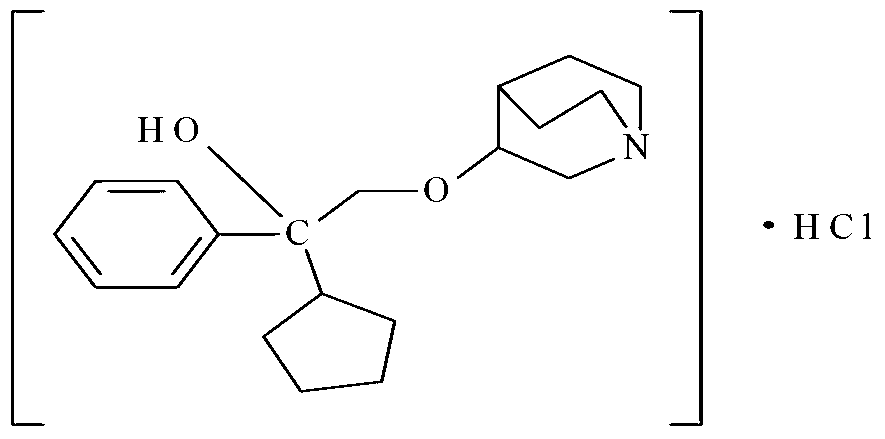
Industrial production method of penehyclidine hydrochloride injection
A technology for penehyclidine hydrochloride and a production method, which are applied in the directions of pharmaceutical formulations, medical preparations containing active ingredients, antidote, etc., can solve the problem of increased damage rate of ampere bottles or vials of washing times, corrosion of stainless steel equipment, and many processes. and other problems, to achieve the effect of clinical efficacy guarantee, safety guarantee, and strong operability.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] The preparation of embodiment 1 penhyclidine hydrochloride injection
[0024] prescription:
[0025] Penhyclidine hydrochloride: 100.0g
[0026] Water for injection: add to 100000ml
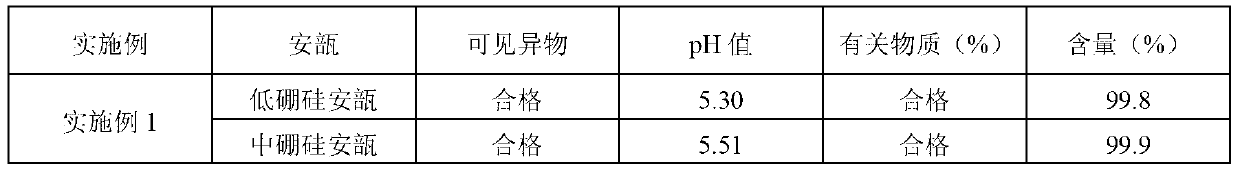
[0027] Preparation process: Weigh 100.0 g of penehyclidine hydrochloride, add 100,000 ml of water for injection to dissolve, add 0.01% (calculated according to the total volume w / v of the preparation) of activated carbon, stir for 10 minutes, and decarburize with a 5um titanium rod for 15 minutes. Adjust the pH to 4.00 with 0.05mol / L hydrochloric acid solution, and the measured pH is 3.99 and the content is 99.6% through the intermediate quality control test. The medicinal liquid is finely filtered with a 0.22um filter membrane, and then filled and sealed in cleaned low-borosilicate ampoules and medium-borosilicate ampoules, sterilized by damp heat at 121°C for 15 minutes, and inspected by light to obtain finished products. The test results of the finished product are shown in Table 1 b...
Embodiment 2
[0030] The preparation of embodiment 2 penhyclidine hydrochloride injection
[0031] prescription:
[0032] Penhyclidine hydrochloride: 100.0g
[0033] Water for injection: add to 100000ml
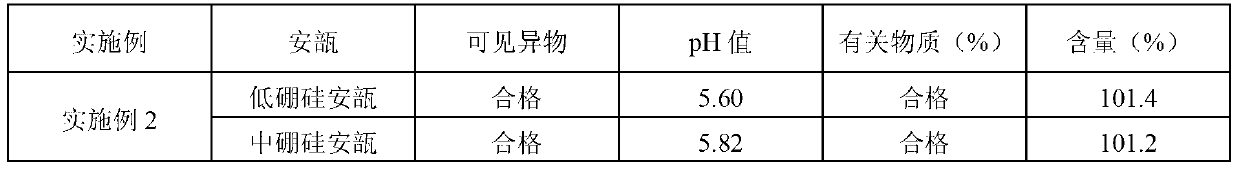
[0034] Preparation process: Weigh 100.0 g of penehyclidine hydrochloride, add 100,000 ml of water for injection to dissolve, add 0.01% (calculated according to the total volume w / v of the preparation) of activated carbon, stir for 10 minutes, and decarburize with a 5um titanium rod for 15 minutes. Use 0.05mol / L hydrochloric acid solution to adjust the pH=4.30, and the measured pH is 4.31 and the content is 101.5% through the intermediate quality control test. The medicinal solution is finely filtered with a 0.22um filter membrane, filled and sealed in cleaned low-borosilicate ampoules and medium-borosilicate ampoules, sterilized by damp heat at 121°C for 15 minutes, and inspected by light to obtain a finished product. The test results of the finished product are shown in Table 2 below: ...
Embodiment 3
[0037] The preparation of embodiment 3 penhyclidine hydrochloride injection
[0038] prescription:
[0039] Penhyclidine hydrochloride: 100.0g
[0040] Water for injection: add to 100000ml
[0041] Preparation process: Weigh 100.0 g of penehyclidine hydrochloride, add 100,000 ml of water for injection to dissolve, add 0.01% (calculated according to the total volume w / v of the preparation) of activated carbon, stir for 10 minutes, and decarburize with a 5um titanium rod for 15 minutes. The pH was adjusted to 4.59 with 0.05mol / L hydrochloric acid solution, and the measured pH was 4.58 through intermediate quality control detection, and the content was 100.2%. The medicinal solution is finely filtered with a 0.22um filter membrane, filled and sealed in cleaned low-borosilicate ampoules and medium-borosilicate ampoules, sterilized by damp heat at 121°C for 15 minutes, and inspected by light to obtain a finished product. The test results of the finished product are shown in Tabl...
PUM
| Property | Measurement | Unit |
|---|---|---|
| acid concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com