Crystal form of penehyclidine hydrochloride racemic mixture I and preparation method thereof
A technology of penehyclidine hydrochloride and racemate, which is applied to the crystal form of penehyclidine hydrochloride racemate I and the field of preparation thereof, and can solve the problem that no reports of penehyclidine hydrochloride racemate have been seen, etc. problem, to improve the stability of the drug, reduce the hygroscopicity, and avoid the effect of deliquescence
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Example 1 Preparation of Penhyclidine Hydrochloride Racemate I Crystal Form of the Present Invention
[0033] Take 25g of 3-(2-hydroxy-2-cyclopentyl-2-phenylethoxy)quinuclidane hydrochloride (also known as penhyclidine hydrochloride), add 75ml of ethanol to reflux to dissolve, then add 25ml of petroleum The ether was naturally cooled, crystals were precipitated, filtered, and the filtrate was set aside for later use. The crystals were penehyclidine hydrochloride racemate I crystal form, 8.4 g in total, and the optical rotation was determined to be 0.
Embodiment 2
[0037] Example 2 Detection of Penhyclidine Hydrochloride Racemate I and II Crystal Forms
[0038] 1. Powder X-ray Diffraction Determination
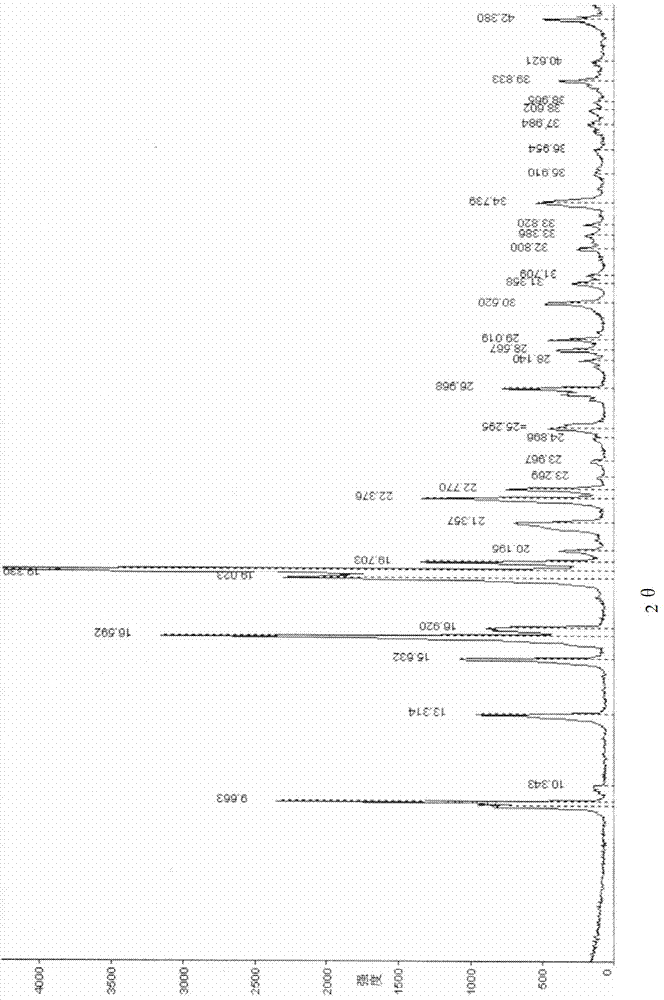
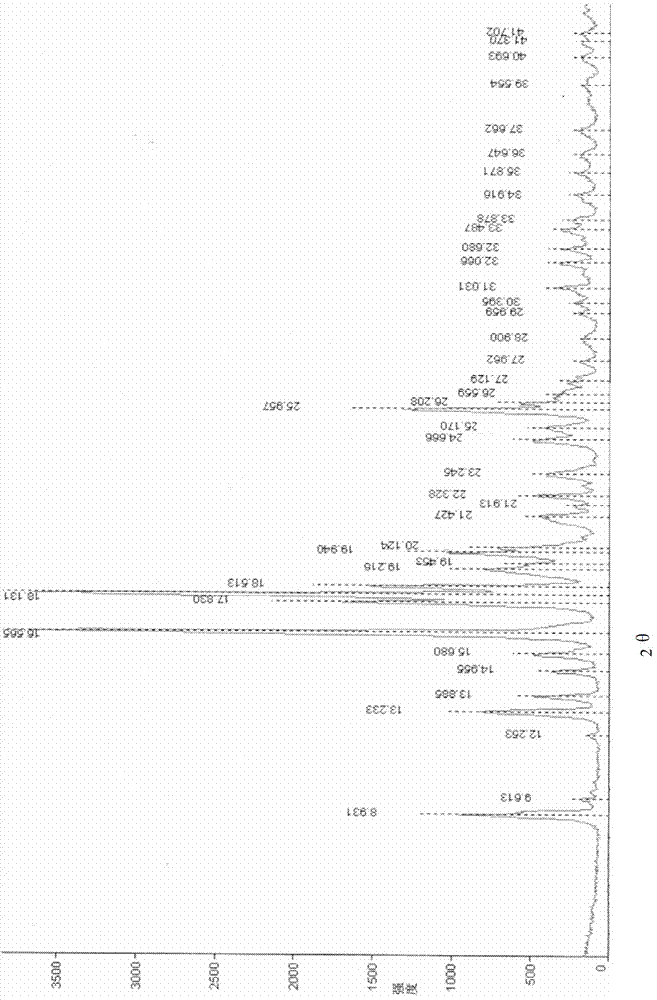
[0039] Powder X-ray diffraction measurement conditions: CuKα line, (monochromator), tube voltage 40KV, tube current 30mA. The powder X-ray diffraction measurement results of the racemate I crystal form obtained in Example 1 are shown in figure 1 , the crystal form of racemate II obtained in the comparative example is shown in figure 2 , see the table below for specific data:
[0040] Table 1
[0041]
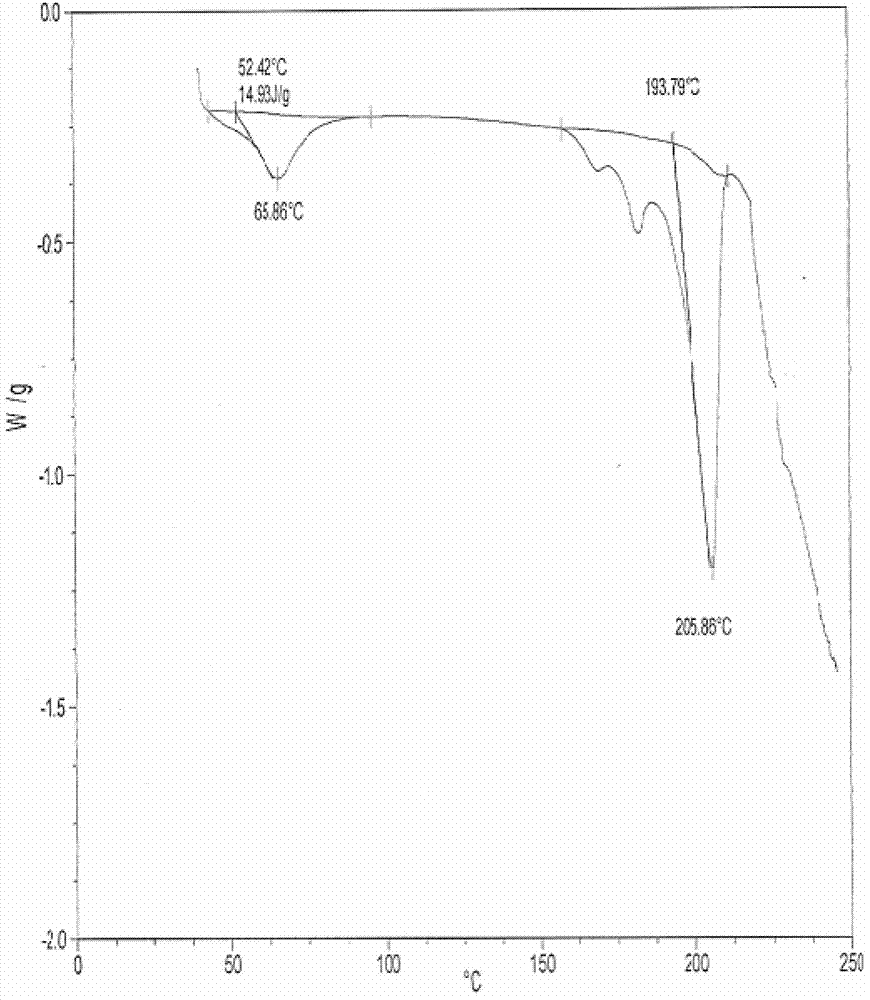
[0042] 2. DSC detection of penehyclidine hydrochloride racemate Ⅰ and Ⅱ crystal forms
[0043] The racemate I and II crystal forms were taken respectively for DSC determination. Among them, the determination results of the racemate I crystal form are shown in image 3 ; Racemate II crystal form determination results see Figure 4 .
[0044] 3. HPLC detection of racemate I crystal form and mixture of two racemate crystal forms...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com