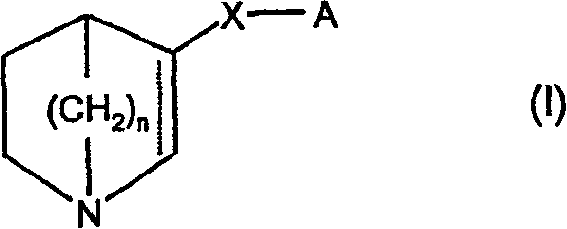Novel quinuclidine derivatives and their use
A technology for quinucidine and derivatives, applied in the field of new quinucidine derivatives and their uses, capable of solving problems such as no reports on the effects of nicotine and/or monoamine receptors
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0188] The present invention is further described in detail with reference to the following examples, which are not intended to limit the scope of the invention claimed in the claims in any way.
[0189] General Notes: All reactions involving air-sensitive reagents or intermediates were performed under nitrogen with anhydrous reagents. Magnesium sulfate was used as a drying agent in the work-up procedure, and the solvent was evaporated under reduced pressure.
[0190] Method A
[0191] (±)-3-(Naphthalen-2-yloxy)-1-aza-bicyclo[2.2.2]octane fumarate
[0192] (Compound A1)
[0193] To 2-naphthol (5.0 g, 34.5 mmol), (±)-3-quinuclidinol (guinuclidinol) (2.94 g, 23.1 mmol), triphenylphosphine (9.0 g, 34.5 mmol) and THF (100 mL) was added to the mixture: diethyl azodicarboxylate (5.4 mL, 34.5 mmol) was added over 30 minutes at room temperature. The reaction mixture was stirred at 50 °C for 20 hours. Aqueous sodium hydroxide solution (100 mL, 1M) was added. The mixture w...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com