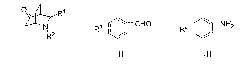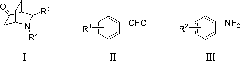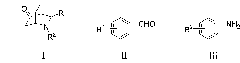Application of fluoro-diphenyl sulfimide as nitrogen heterocyclic Diels-Alder reaction catalyst
A bisbenzenesulfonimide and catalyst technology, which is applied in the new application field of fluorobisbenzenesulfonimide, can solve the problems that have not been seen in application reports, and achieve the advantages of simple and fast operation, low environmental pollution and low production cost Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0016] In order to make the object, technical solution and advantages of the present invention clearer, preferred embodiments of the present invention are described in detail below.
[0017] The fluorinated bisbenzenesulfonimide used in the preferred embodiment is purchased from Tianjin Jiuri Chemical Industry Co., Ltd., and the reaction starting raw material cyclohexenone, substituted benzaldehyde and substituted aniline and other reagents are commercial reagents and have not been used. Further purification. The structure of the product was confirmed by NMR spectroscopy. In order to facilitate the comparison of results, the room temperature in the method was controlled at 25±1°C.
[0018] 1. Catalyst type and reaction condition optimization for aza Diels-Alder reaction
[0019] The present invention first takes cyclohexenone, nitrobenzaldehyde and p-methoxyaniline as model compound, the kind of catalyst (N-F electrophilic fluorinating reagent) and reaction conditions (react...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com