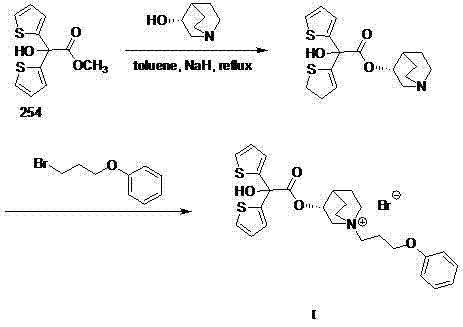
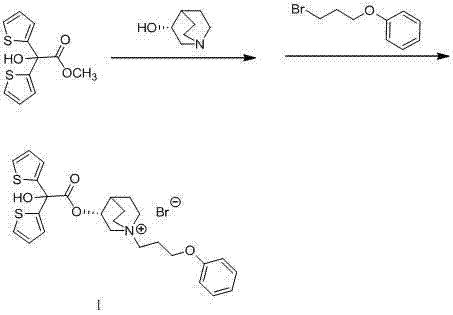
Technology for preparing aclidinium bromide employing one-pot process
A kind of aclidinium bromide, process technology, applied in the field of one-pot preparation of aclidinium bromide, can solve the problems of cumbersome operation, complicated post-processing, long reaction time, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0015] Add 50mL of toluene into a 100mL three-necked flask, and then add (R)-3-quinuclidinol (1.52g, 12mmol), sodium hydride (0.60g, 15mmol), methyl 2,2-dithienyl glycolate (2.54 g, 10mmol), N 2 Under protection, heat to reflux for 4 hours, cool to room temperature; then add 3-phenoxypropyl bromide (2.15 g, 10 mmol) into the same reactor, heat and stir the reaction, and control the reaction temperature at 60-65°C, TLC tracking, until the thin-layer plate shows that the component point of the intermediate product disappears, cool to room temperature, add 10 mL of water to quench, separate the liquid, and distill the organic phase under reduced pressure. The residue is pulped with petroleum ether, and a white solid is obtained by suction filtration. Crystallized to obtain 1.9 g of white powder with a yield of 33.7%. m.p.227.5-230.0℃; 1 H NMR (400 MHz, DMSO) δppm 1.67(m, 2H), 1.97(m, 2H), 2.11(m, 2H), 2.32(m, 1H), 3.21(m, 1H), 3.45(m, 6H), 4.01(m, 3H), 5.16(m, 1H), 6.92(m, 3...
Embodiment 2
[0017] Add 50mL xylene into a 100mL three-necked flask, and then add (R)-3-quinuclidinol (1.52g, 12mmol), sodium hydride (0.60g, 15mmol), methyl 2,2-dithienyl glycolate ( 2.54g, 10mmol), N 2 Under protection, heat to reflux for 4 hours, cool to room temperature; then add 3-phenoxypropyl bromide (2.15g, 10mmol) into the same reactor, heat and stir the reaction, control the reaction temperature at 60-65°C, TLC Track until the thin-layer plate shows that the component point of the intermediate product disappears, cool to room temperature, add 10 mL of water to quench, separate the liquid, distill the organic phase under reduced pressure, beat the residue with petroleum ether, filter with suction to obtain a white solid, and recrystallize from methanol , to obtain white powder 2.0g, productive rate 35.5%. m.p. 226.5-229.0°C.
Embodiment 3
[0019] Add 50mL of ethylene glycol dimethyl ether into a 100mL three-necked flask, and then add (R)-3-quinuclidinol (1.52g, 12mmol), sodium hydride (0.60g, 15mmol), and 2,2-dithienyl ethanol Acid methyl ester (2.54g, 10mmol), N 2 Under protection, heat to reflux for 4 hours, cool to room temperature; then add 3-phenoxypropyl bromide (2.15g, 10mmol) into the same reactor, heat and stir the reaction, control the reaction temperature at 60-65°C, TLC Track until the thin-layer plate shows that the component point of the intermediate product disappears, cool to room temperature, add 10 mL of water to quench, separate the liquid, distill the organic phase under reduced pressure, beat the residue with petroleum ether, filter with suction to obtain a white solid, and recrystallize from methanol , to obtain 2.4 g of white powder, yield 42.4%. m.p. 228.0-229.5°C.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com