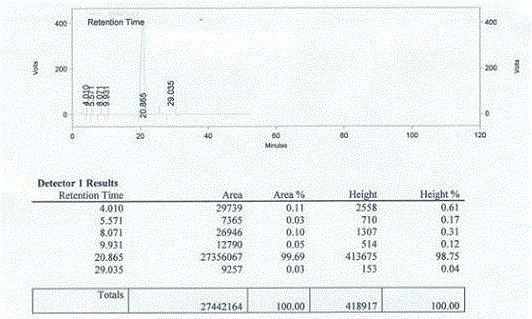
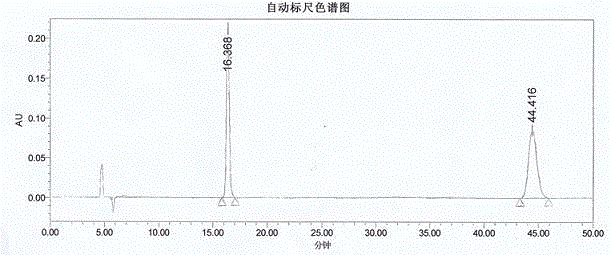
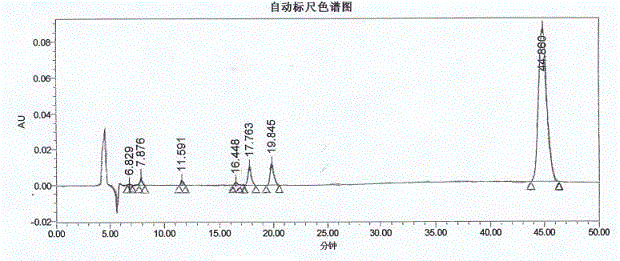
Method for measuring optical purity of (R)-3-quinuclidinol
A technology of optical purity and determination method, applied in the field of medicine and chemical industry, can solve the problems of difficult chiral chromatographic separation, lack of groups, inapplicability, etc., and achieve the effect of good peak purity and enhanced identification ability.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] The method for measuring the optical purity of R-3-quinuclidinol is as follows:
[0050] (A) Sample preparation:
[0051] (A1) Preparation of (RS)-3-quinuclidinyl p-toluenesulfonate:
[0052] Add 30 mL of DCM, 5 g of 3-quinuclidinol and 11.5 g of TEA to a 100 mL reaction flask, cool to 0°C, and slowly add a solution of 11 g of p-toluenesulfonyl chloride and 30 mL of DCM at 0-5°C while stirring, and finish dropping. Slowly rise to room temperature, continue to stir the reaction until TLC detects the reaction is complete, cool to 0°C, slowly add 30 mL of water with stirring, separate the layers, wash the organic layer once with 50 mL of water, wash once with 50 mL of saturated sodium chloride solution, and dry. After filtration, the filtrate was concentrated until no liquid dripped out, and purified by column chromatography to obtain a slightly yellow solid, namely (RS)-3-quinuclidinyl p-toluenesulfonate, 8 g in total, with a yield of 68%;
[0053] (A2) Preparation of (R)-3-quin...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Aperture | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com