Method for synthesizing palonosetron hydrochloride
A synthesis method and hydrochloric acid technology are applied in the new synthesis field of palonosetron hydrochloride, can solve the problems of complicated post-processing, difficult purification, dangerous reagents and the like, and achieve the effects of easy separation and purification, low price and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
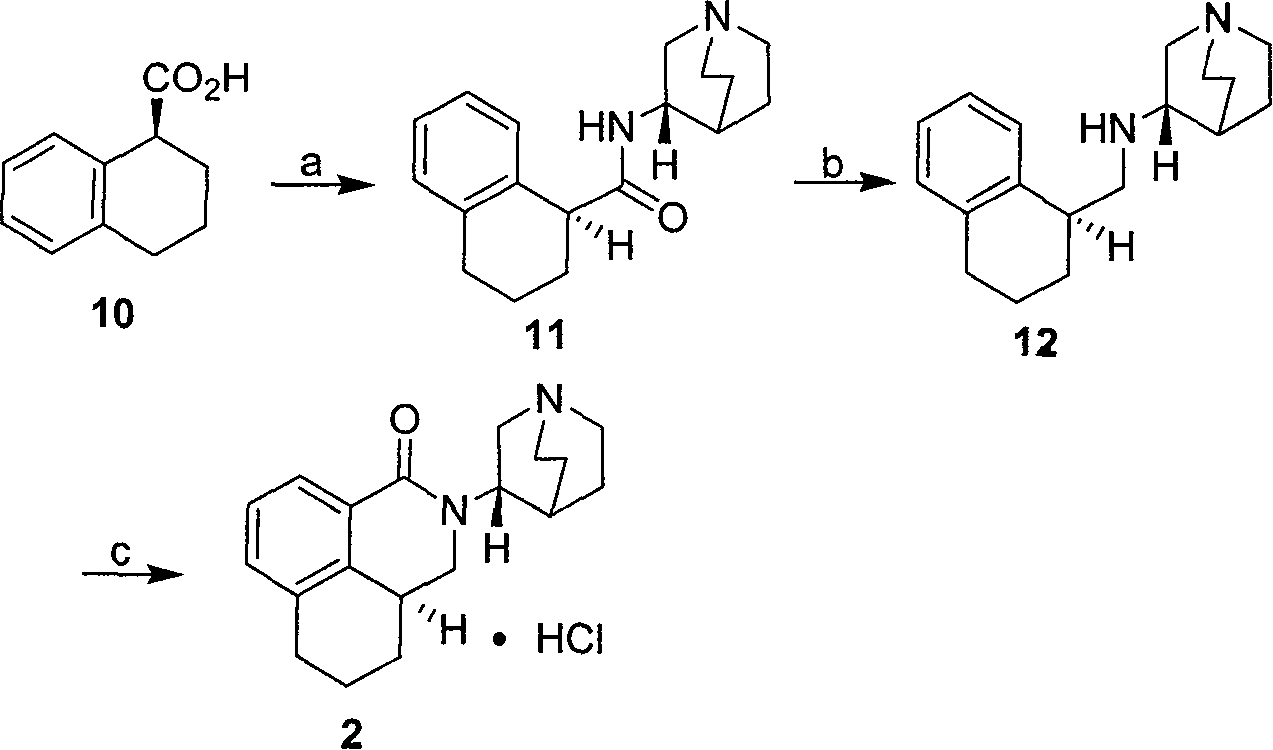
[0053] [embodiment 1] the preparation of formula (11) compound (S, S)-quinuclidine tetrahydronaphthalene carboxamide
[0054] Add (S)-tetrahydronaphthoic acid (0.17mol), thionyl chloride (0.18mol) and toluene into a 250mL reaction flask, then heat up to 50°C for 1 hour, and drop (S)-3-amino - Quinucidine (0.17mol), reacted at 50° C. for 1 hour, sampling for thin-layer chromatography analysis showed that the reaction was complete, and the reaction solution changed from clear to turbid at the same time. Cool to room temperature, add 0.3 L of water and 13.5 mL of 50% sodium hydroxide solution, extract with ethyl acetate, and dry over anhydrous sodium sulfate to obtain a white solid. The solvent is distilled off under reduced pressure to obtain a white solid with a yield of 93%. TLC (plate chromatography) shows that 11 is a single compound: (S, S)-quinuclidine tetrahydronaphthalene carboxamide compound melting point (Mp) is: 190-191 ° C; optical rotation is [α] D 20 = -56.4 (c 1.0...
Embodiment 2
[0055] [embodiment 2] the preparation of formula (11) compound (S, S)-quinuclidine tetrahydronaphthalene carboxamide
[0056] Add (S)-tetrahydronaphthoic acid (0.17mol), thionyl chloride (0.26mol) and toluene into a 250mL reaction flask, then heat up to 80°C for 6 hours, and dropwise add (S)-3-amino - Quinucidine (0.26mol), reacted at 80° C. for 3 hours, sampling for thin-layer chromatography analysis showed that the reaction was complete, and the reaction solution changed from clear to turbid at the same time. Cool to room temperature, add 0.3 L of water and 13.5 mL of 50% sodium hydroxide solution, extract with ethyl acetate, and dry over anhydrous sodium sulfate to obtain a white solid. The solvent is distilled off under reduced pressure to obtain a white solid with a yield of 86%.
Embodiment 3
[0057] [embodiment 3] the preparation of formula (12) compound (S, S)-tetralin methyl quinuclidine
[0058] (S, S)-quinuclidine tetrahydronaphthalene carboxamide compound (0.16mol) and sodium borohydride (0.64mol) and tetrahydrofuran 0.9L are added in the reaction bottle of 2000ml, and temperature is controlled between-10~15 ℃, then Add boron trifluoride ether solution (0.84mol) slowly, dropwise is completed, warm up to room temperature, react for 30 minutes, the temperature rises to 60°C and react for 2 hours, sampling for thin-layer chromatography analysis shows that the reaction is complete, and the reaction solution From cloudy to clear. Cool to below 20°C, slowly add 2N hydrochloric acid (0.69L) solution, concentrate to 0.75L, add 50% potassium hydroxide (271g) under cooling, extract with ethyl acetate, dry over anhydrous sodium sulfate, filter and concentrate to obtain an oil The product (S, S)-tetrahydronaphthylmethylquinuclidine, the total yield>98%. TLC (plate chrom...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com