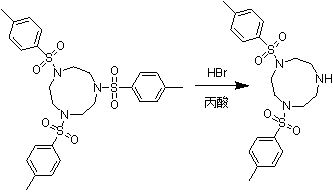
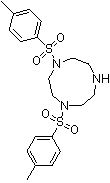
Synthesis method of 1,4-bis(p-toluenesulfonyl)trinitrocyclononane
A technology of benzoyl triazacyclononane and p-toluenesulfonyl group is applied in the field of synthesis of 1,4-bis-triazacyclononane, and can solve the problems of low reaction yield, low production efficiency, waste acid and the like, Achieve the effects of avoiding side reactions, low production costs, and simple process control
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
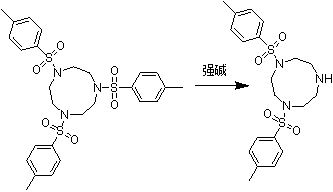
[0026] In a 500ml four-necked flask equipped with an electric stirrer, a thermometer, a reflux condenser, and an oil bath heating pot, add 10g (0.018mol) of 1,4-bis(p-toluenesulfonyl)-7-benzoyl triazine Cyclononane, 5g (0.045mol) potassium tert-butoxide, 300ml tert-butanol, after adding the above materials, start to stir and heat up.
[0027] When the system was refluxed, start timing the heat preservation reaction. After 36 hours of heat preservation reaction, cool to room temperature and filter. The filter cake was washed with a large amount of water until the pH was neutral. The filter cake was dried to obtain 7.11 g. The liquid chromatography content is 96.75%, based on 1,4-bis(p-toluenesulfonyl)-7-benzoyltriazacyclononane, the molar yield is 90.39%.
Embodiment 2
[0029] In a 500ml four-necked flask equipped with an electric stirrer, a thermometer, a reflux condenser, and an oil bath heating pot, add 10g (0.018mol) of 1,4-bis(p-toluenesulfonyl)-7-benzoyl triazine Cyclononane, 3g (0.031mol) sodium tert-butoxide, 300ml tert-butanol, after adding the above materials, start to stir and heat up.
[0030] When the system was refluxed, start timing the heat preservation reaction. After 36 hours of heat preservation reaction, cool to room temperature and filter. The filter cake was washed with a large amount of water until the pH was neutral. The filter cake was dried to obtain 6.6 g. The liquid chromatography content is 95.17%, based on 1,4-bis(p-toluenesulfonyl)-7-benzoyltriazacyclononane, the molar yield is 83.91%.
Embodiment 3
[0032] In a 500ml four-necked flask equipped with an electric stirrer, a thermometer, a reflux condenser, and an oil bath heating pot, add 10g (0.018mol) of 1,4-bis(p-toluenesulfonyl)-7-benzoyl triazine Cyclononane, 20g (0.088mol) sodium ethoxide ethanol solution with a mass fraction of 30%, and 300ml of ethanol, after adding the above materials, start to stir and heat up.
[0033] When the system was refluxed, start timing the heat preservation reaction. After 12 hours of heat preservation reaction, cool to room temperature and filter. The filter cake was washed with a large amount of water until the pH was neutral. The filter cake was dried to obtain 3.2 g. The liquid chromatography content is 88.55%, based on 1,4-bis(p-toluenesulfonyl)-7-benzoyltriazacyclononane, the molar yield is 40.68%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com