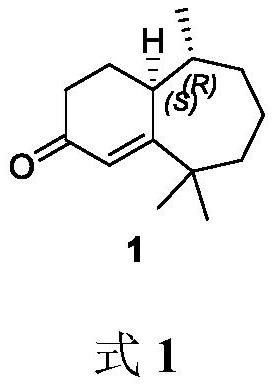
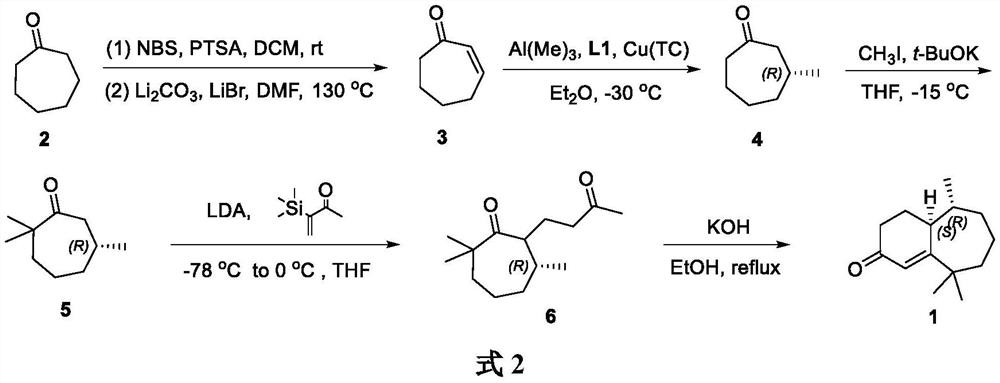
A kind of method for synthesizing flea beetle aggregation pheromone
A technology for gathering pheromones and flea beetles, which is applied in the field of green pesticides and can solve problems such as low total yield, difficulty in scaling up, and lengthy synthetic routes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Synthesis of 2-cyclohepten-1-one (3)
[0023] Cycloheptanone (5.608 g, 50.00 mmol) was added to a 500 mL single-neck flask, and anhydrous DCM (50 mL) was added. P-toluenesulfonic acid monohydrate (85.0 mg, 0.50 mmol) was slowly added, and the reaction was carried out at room temperature for 10 min. Under ice-cooling, NBS (9.788 g, 55.00 mmol) was slowly added, the temperature was gradually raised to room temperature, and the reaction was carried out at room temperature for 16 h to stop the reaction. Under ice-cooling, saturated sodium thiosulfate solution (40 mL) was added, and the solution was separated. The aqueous phase was extracted with DCM (3 x 100 mL) and the organic phases were combined. Anhydrous Na for organic phase 2 SO 4 Dry and remove the solvent under reduced pressure. Finally, it was purified by silica gel column chromatography (petroleum ether:ethyl acetate 10:1) to obtain the crude bromocycloheptanone.
[0024] Under the protection of argon, lithi...
Embodiment 2
[0026] Synthesis of (R)-3-methylcycloheptanone (4)
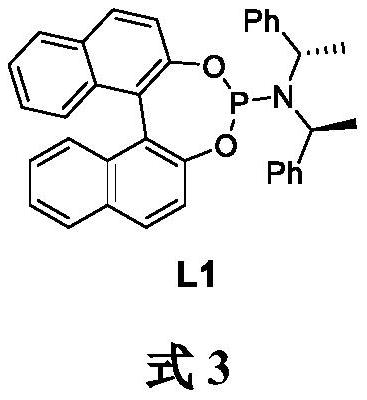
[0027] Under argon, Cu(TC) (28.6 mg, 0.15 mmol), ligand L1 (307.8 mg, 0.6 mmol) and ultra-dry Et 2 O (10 mL) was added to a 100 mL Shrek bottle, and the reaction was stirred for 1 h. Cool to -30°C and add trimethylaluminum (18 mL, 1 M in ether, 18 mmol) dropwise. The reaction was carried out at -30 °C for 1 h, then 2-cyclohepten-1-one (3) (1.100 g, 10 mmol) was added dropwise within 1 h, and the reaction was carried out at -30 °C for 6 h to stop the reaction. The reaction was quenched with ice (5.00 g) and the layers were separated. Et for water phase 2 O (3 x 30 mL) extraction, combined organic phases. Anhydrous Na for organic phase 2 SO 4Dry and remove the solvent under reduced pressure. Finally, it was purified by silica gel column chromatography (petroleum ether:diethyl ether 30:1) to obtain (R)-3-methylcycloheptanone (4) as a colorless oil (1.00 g, yield 85%, optical purity 90%) .
[0028] See formula 3 for the...
Embodiment 3
[0031] Synthesis of (R)-2,2,6-trimethylcycloheptanone (5)
[0032] Under argon protection, potassium tert-butoxide (1.137 g, 10.15 mmol) and anhydrous THF (20 mL) were added to a 100 mL Shrek bottle, and stirred uniformly. Then, a solution of chiral methylcycloheptanone 4 (800 mg, 6.34 mmol) in THF (5 mL) was added dropwise, the temperature was lowered to 0° C., and the reaction was stirred for 1 h. Continue to cool down to -15°C, add iodomethane (1.800 g, 12.68 mmol) dropwise, and react at -15°C for 15 min to stop the reaction. Saturated aqueous ammonium chloride solution (10 mL) was added to quench the reaction, the layers were separated, and the aqueous phase was treated with Et 2 Extract with O (3 x 20 mL) and combine the organic phases. Anhydrous Na for organic phase 2 SO 4 Dry and remove the solvent under reduced pressure. Finally, it was purified by silica gel column chromatography (petroleum ether:diethyl ether 80:1) to obtain trimethylcycloheptanone 5 (0.780 g, 8...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com