Camphor-based ketoxime fluorescent probe for detecting hypochlorous acid as well as preparation method and application of camphor-based ketoxime fluorescent probe
A technology for detecting hypochlorous acid and camphor-based ketoxime, which is applied in oxime preparation, fluorescence/phosphorescence, chemical instruments and methods, etc., to achieve simple synthesis, good application value, and high sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
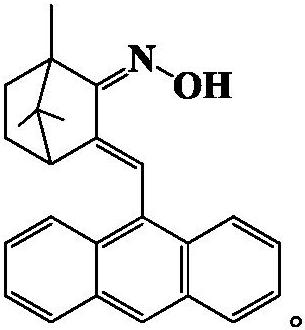
[0018] The preparation of 3-(9-anthracene methylene)-1,7,7-trimethylbicyclo[2.2.1]heptan-2-one oxime, the reaction formula is as follows:
[0019]
[0020] Specific steps are as follows:
[0021] Preparation of 3-(9-anthracenemethylene)-1,7,7-trimethylbicyclo[2.2.1]heptan-2-one oxime:
[0022] 1.5mmol 3-(9-anthracene methylene)-1,7,7-trimethylbicyclo[2.2.1]heptan-2-one, 6.0mmol hydroxylamine hydrochloride, 7.5mmol sodium hydroxide, 30mL absolute ethanol In a three-necked flask equipped with a stirrer, the reaction was refluxed at 80°C for 48h. After the solvent was recovered from the reaction solution by distillation, the crude product of 3-(9-anthracene methylene)-1,7,7-trimethylbicyclo[2.2.1]heptan-2-one oxime was obtained, and then passed through a silica gel chromatography column ( Petroleum ether: ethyl acetate = 4:1) chromatography and methanol recrystallization to obtain yellow powder 3-(9-anthracene methylene)-1,7,7-trimethylbicyclo[2.2.1]hept-2- Ketoxime, the yi...
Embodiment 2
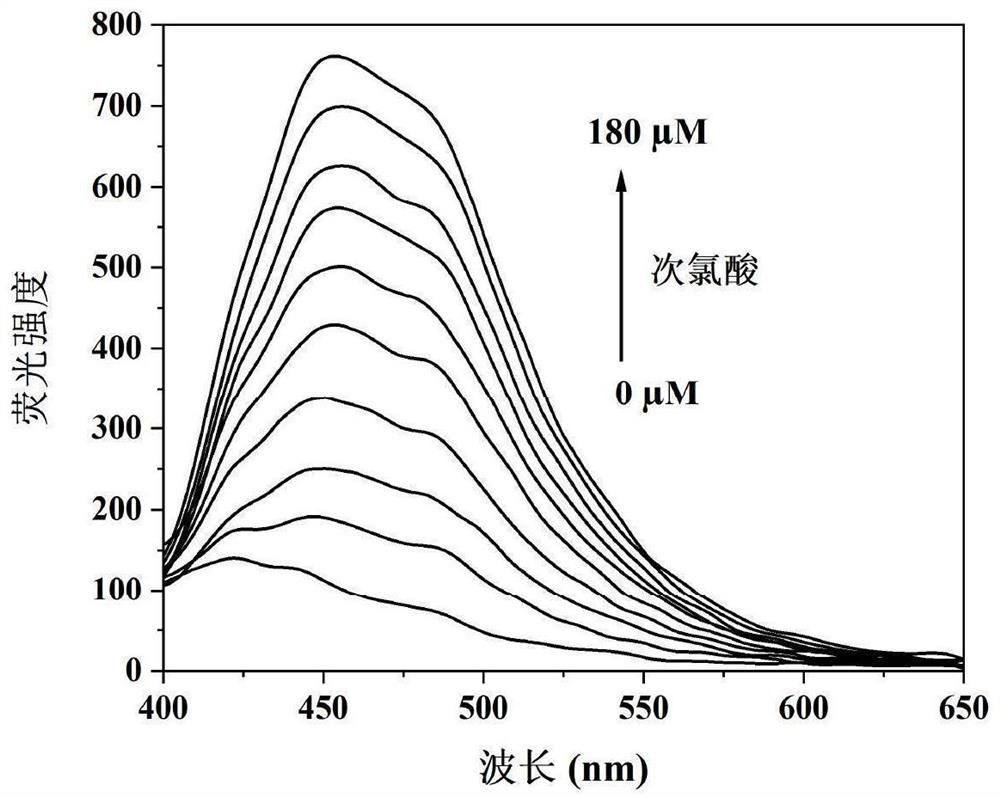
[0024] Prepare 3-(9-anthracene methylene)-1,7,7-trimethylbicyclo[2.2.1]heptan-2-one oxime to 1.5×10 -6 M's PBS buffer solution, dissolve cysteine in PBS buffer solution to make the concentration 0, 1.0×10 -5 , 2.0×10 -5 , 4.0×10 -5 , 6.0×10 -5 , 8.0×10 -5 , 10.0×10 -5 , 12.0×10 -5 , 14.0×10 -5 , 16.0×10 -5 , 18.0×10 -5 M's solution. Adopt fluorescence spectrometric titration to measure on the fluorescence spectrophotometer under the hypochlorous acid action of different concentrations 3-(9-anthracene methylene)-1,7,7-trimethylbicyclo[2.2.1]heptane Fluorescence emission spectra of 2-ketoximes, such as figure 1 shown. The results show that with the gradual increase of the concentration of hypochlorous acid in the solution, the fluorescence emission intensity of the probe at 453nm is gradually enhanced, thus indicating that the compound can be used as a fluorescent probe for sensitively detecting the content of hypochlorous acid in the solution.
Embodiment 3
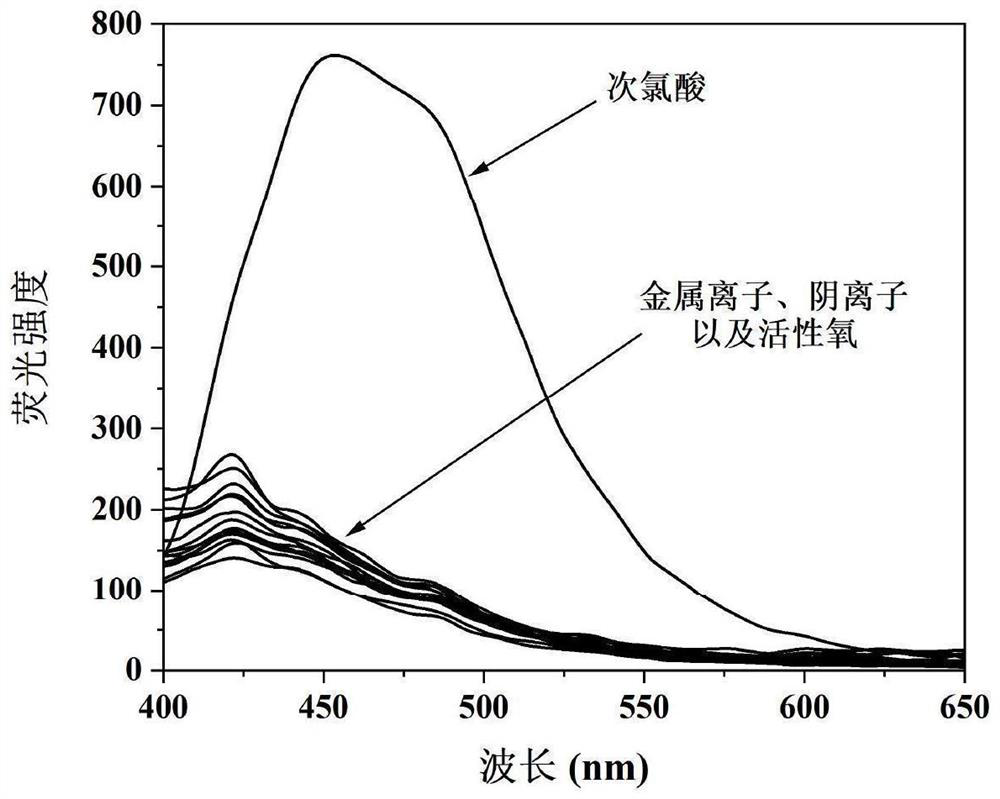
[0026] Prepare 3-(9-anthracene methylene)-1,7,7-trimethylbicyclo[2.2.1]heptan-2-one oxime to 1.5×10 -6 M's PBS buffer solution, and different metal ions, anions and active oxygen were dissolved in PBS buffer to prepare a concentration of 1.8×10 - 4 M's solution. Fluorescence spectrometry was used to measure the concentration of 3-(9-anthracene methylene)-1,7,7-trimethylbicyclo[2.2.1]heptane under the action of different metal ions, anions and active oxygen on a fluorescence spectrophotometer. - Fluorescence emission spectra of 2-ketoximes, such as figure 2 shown. The results show that the addition of hypochlorous acid will significantly increase the fluorescence emission intensity of the probe at 453nm, while the addition of other metal ions (Na + , Cu 2+ , Zn 2+ , Mg 2+ , Al 3+ , Cd 2+ ), anion (F - , SO 3 2- , CO 3 2- , AcO - , NO 2 - ) and reactive oxygen species ( 1 o 2 , H 2 o 2 , ONOO - ) and other reference comparison observations, the fluorescenc...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com