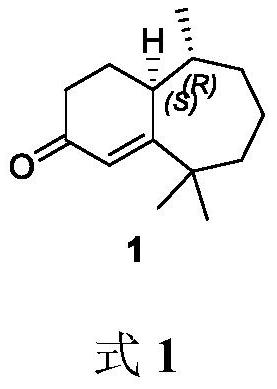
Method for synthesizing flea beetle aggregation pheromone
A technology for gathering pheromones and flea beetles, which is applied in the field of green pesticides and can solve problems such as low total yield, difficulty in scaling up, and lengthy synthetic routes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
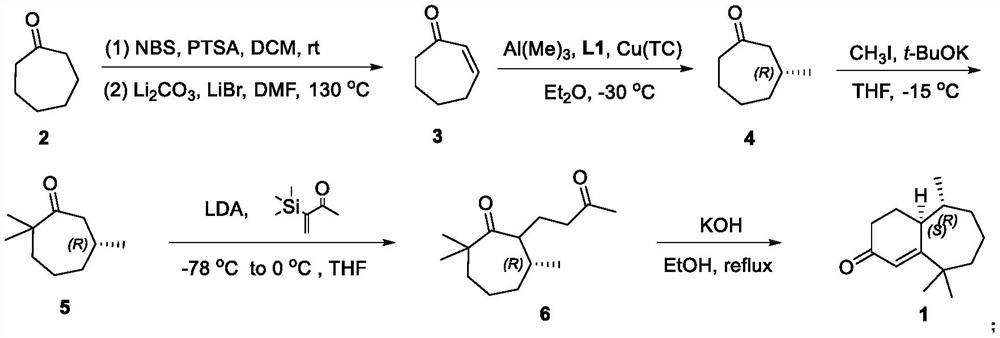
[0022] Synthesis of 2-cyclohepten-1-one (3)
[0023] Cycloheptanone (5.608 g, 50.00 mmol) was added to a 500 mL one-necked flask, and anhydrous DCM (50 mL) was added. Add p-toluenesulfonic acid monohydrate (85.0 mg, 0.50 mmol) slowly and react at room temperature for 10 min. Under cooling in an ice bath, NBS (9.788 g, 55.00 mmol) was added slowly, the temperature was gradually raised to room temperature, and the reaction was carried out at room temperature for 16 h, then the reaction was stopped. Under cooling in an ice bath, a saturated solution of sodium thiosulfate (40 mL) was added, and the layers were separated. The aqueous phase was extracted with DCM (3 x 100 mL), and the organic phases were combined. Anhydrous Na for organic phase 2 SO 4 Dry and remove solvent under reduced pressure. Finally, it was purified by silica gel column chromatography (petroleum ether: ethyl acetate 10:1) to obtain the crude product of bromocycloheptanone.
[0024] Under argon protection...
Embodiment 2
[0026] Synthesis of (R)-3-methylcycloheptanone (4)
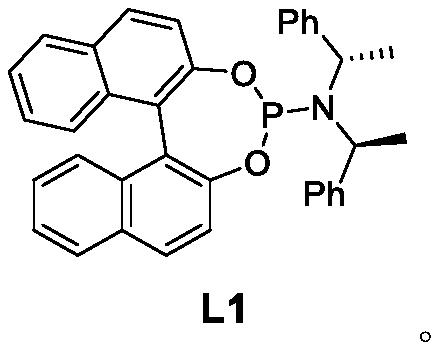
[0027] Under argon protection, Cu(TC) (28.6mg, 0.15mmol), ligand L1 (307.8mg, 0.6mmol) and ultra-dry Et 2 O (10 mL) was added into a 100 mL Shrek bottle, and the reaction was stirred for 1 h. The temperature was lowered to -30°C, and trimethylaluminum (18 mL, 1M ether solution, 18 mmol) was added dropwise. React at -30°C for 1 h, then add 2-cyclohepten-1-one (3) (1.100 g, 10 mmol) dropwise within 1 h, react at -30°C for 6 h, and stop the reaction. The reaction was quenched with ice (5.00 g) and the layers were separated. Et for aqueous phase 2 O (3×30 mL) was extracted, and the organic phases were combined. Anhydrous Na for organic phase 2 SO 4Dry and remove solvent under reduced pressure. Finally, it was purified by silica gel column chromatography (petroleum ether: ether 30:1) to obtain (R)-3-methylcycloheptanone (4) (1.00g, yield 85%, optical purity 90%) as a colorless oil .
[0028] See Formula 3 for the structu...
Embodiment 3
[0031] Synthesis of (R)-2,2,6-trimethylcycloheptanone (5)
[0032] Under argon protection, potassium tert-butoxide (1.137 g, 10.15 mmol) and anhydrous THF (20 mL) were added to a 100 mL Shrek bottle, and stirred evenly. Then a solution of chiral methylcycloheptanone 4 (800mg, 6.34mmol) in THF (5mL) was added dropwise, the temperature was lowered to 0°C, and the reaction was stirred for 1h. Continue to cool down to -15°C, add iodomethane (1.800g, 12.68mmol) dropwise, react at -15°C for 15min, and stop the reaction. Add saturated aqueous ammonium chloride solution (10mL) to quench the reaction, separate the layers, and wash the aqueous phase with Et 2 O (3×20 mL) was extracted, and the organic phases were combined. Anhydrous Na for organic phase 2 SO 4 Dry and remove solvent under reduced pressure. Finally, it was purified by silica gel column chromatography (petroleum ether: ether 80:1) to obtain trimethylcycloheptanone 5 (0.780 g, yield 80%) as a colorless oil.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com