Camphor-based enhanced fluorescent probe for detecting Fe2+ as well as preparation method and application of camphor-based enhanced fluorescent probe
A fluorescent probe and enhanced technology, applied in the direction of fluorescence/phosphorescence, chemical instruments and methods, luminescent materials, etc., can solve problems such as interference and lack of selectivity, and achieve fast response, high sensitivity, and easy synthesis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] N,N-Dimethyl-4-(3-(4,7,7-trimethyl-3-oxobicyclo[2.2.1]hept-2-enyl)prop-1-en-1-yl) The synthesis of aniline oxide, reaction formula is as follows:
[0028]
[0029] Specific steps are as follows:
[0030] 1) Preparation of 3-(3-(4-(dimethylamino)phenyl)allylidene)-1,7,7-trimethylbicyclo[2.1.1]heptan-2-one:
[0031]Add 10mmol of camphor, 12mmol of 4-dimethylaminocinnamaldehyde, 30mL of tert-butanol and 20mmol of potassium tert-butoxide into a dry three-necked flask in sequence, and stir and reflux for 12h. After the reaction solution was distilled off under reduced pressure to remove the solvent tert-butanol, ethyl acetate was added, and then washed with saturated brine until neutral, and the organic phase was dried over anhydrous sodium sulfate, filtered, and concentrated to obtain 3-(3-(4 -(Dimethylamino)phenyl)allylidene)-1,7,7-trimethylbicyclo[2.1.1]heptan-2-one crude product, recrystallized from anhydrous methanol to obtain orange-red powder 3-(3-(4-(dimethylam...
Embodiment 2
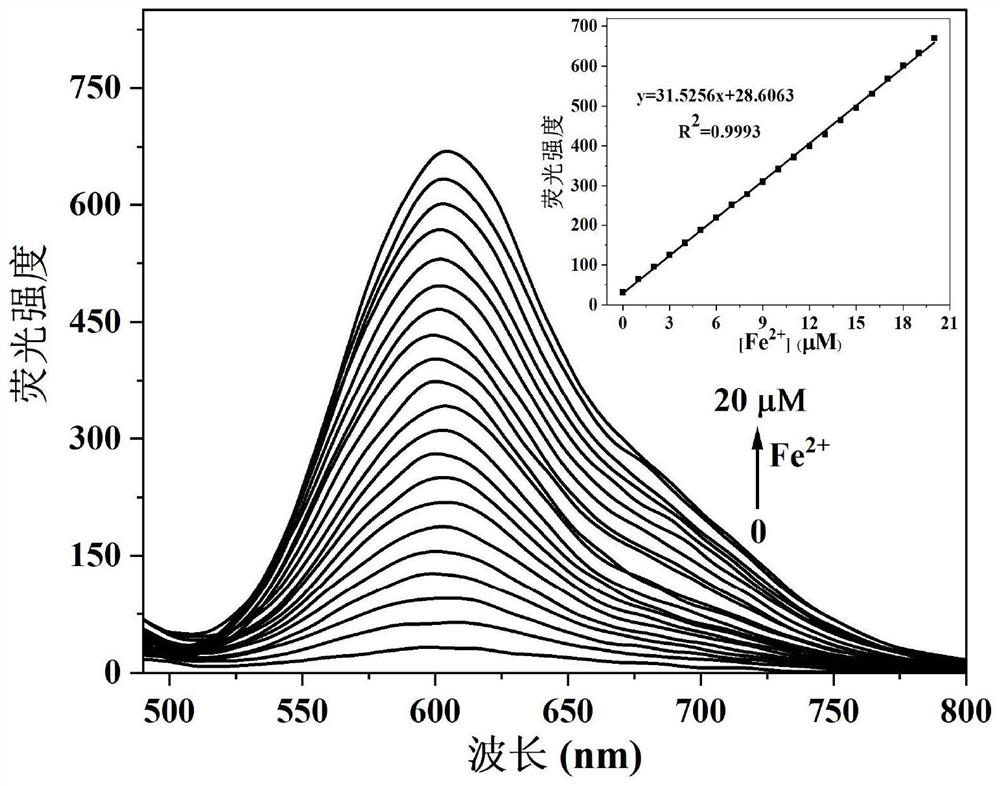
[0035] N, N-dimethyl-4-(3-(4,7,7-trimethyl-3-oxobicyclo[2.2.1]hept-2-enyl)prop-1-en-1-yl ) aniline oxide was dissolved in PBS / EtOH (v / v=8 / 2) buffer to prepare 1×10 -5 M concentration of the probe solution, the Fe 2+ Dissolved in PBS / EtOH (v / v=8 / 2) buffer solution to prepare concentration of 0, 1×10 -6 , 2×10 -6 , 3×10 -6 , 4×10 -6 , 5×10 -6 , 6×10 -6 , 7×10 -6 , 8×10 -6 , 9×10 -6 , 10×10 -6 , 11×10 -6 , 12×10 -6 , 13×10 -6 , 14×10 -6 , 15×10 -6 , 16×10 -6 , 17×10 -6 , 18×10 -6 , 19×10 -6 , 20×10 -6 M's solution. Different concentrations of Fe were measured by a standard titration method under a fluorescence spectrophotometer 2+ p-N,N-dimethyl-4-(3-(4,7,7-trimethyl-3-oxobicyclo[2.2.1]hept-2-enyl)prop-1-en-1-yl ) Fluorescence emission spectrum of aniline oxide, such as figure 1 shown. The results showed that within the concentration range of 0-20μM, with the Fe in the system 2+ As the concentration increased, the solution changed from colorless to red fl...
Embodiment 3
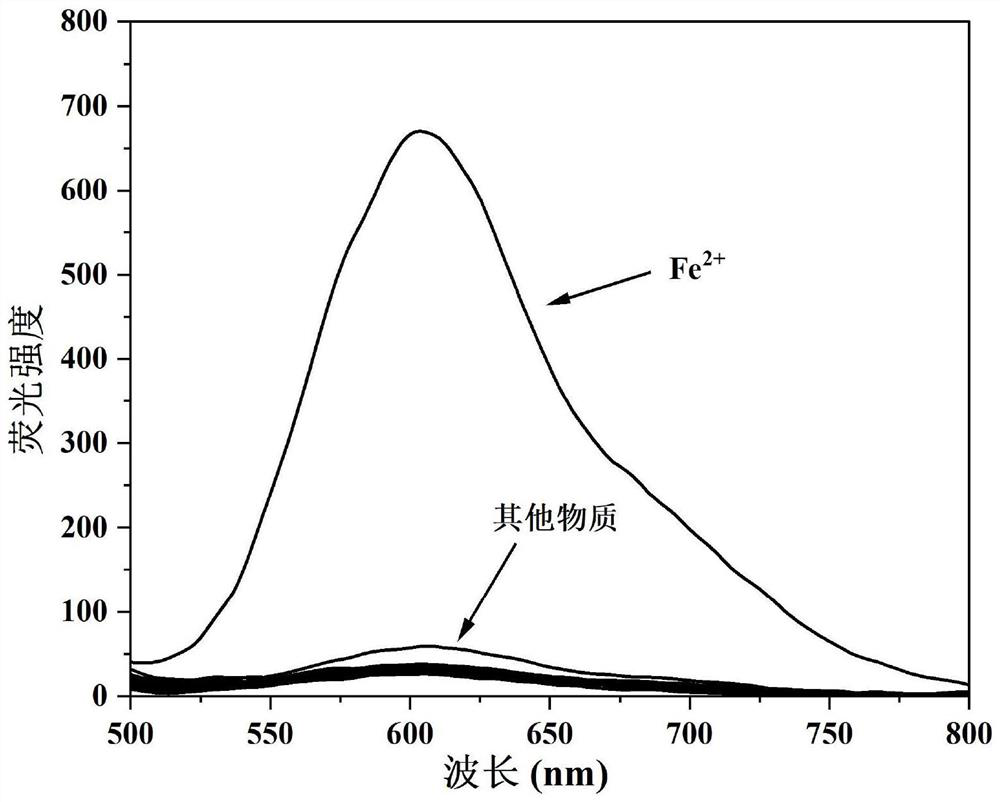
[0037] N, N-dimethyl-4-(3-(4,7,7-trimethyl-3-oxobicyclo[2.2.1]hept-2-enyl)prop-1-en-1-yl ) aniline oxide was dissolved in PBS / EtOH (v / v=8 / 2) buffer to prepare 1×10 -5 The probe solution of M concentration is prepared by dissolving different metal ions, anions and biologically relevant substances in PBS / EtOH (v / v=8 / 2) buffer solution to make 100×10 -6 M concentration solution, Fe 2+ Formulated to 20×10 -6 M concentration solution. Using standard titration method to measure the effect of different metal ions and biologically relevant species on N,N-dimethyl-4-(3-(4,7,7-trimethyl-3-oxobicyclo[ 2.2.1] The fluorescence emission spectrum of hept-2-enyl)prop-1-en-1-yl)aniline oxide, as figure 2 shown. The results showed that the addition of Fe to the probe solution 2+ After the red fluorescence is enhanced, and the addition of K + 、Na + , Mg 2+ , Ca 2+ 、Cu 2+ , Zn 2+ 、Al 3+ 、Ni + 、Cs 2+ , Sn 2+ 、Cr 3+ 、Cd 2+ , Mn 2+ , La 3+ 、Co 2+ , Hg 2+ 、Ag + , Pb 2+ 、Ce 4...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com