Chiral N-heterocyclic carbene catalyst as well as preparation method and application thereof
A heterocyclic carbene and catalyst technology, applied in the field of organic chemistry applications, can solve problems such as poor compatibility and low reactivity, and achieve good catalytic activity, strong operability, and broad market application prospects
Active Publication Date: 2021-09-03
CHENGDU UNIV
View PDF4 Cites 1 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
The technical effect of this new type of catalyst described by this patented method can be improved efficiency or productivity compared to previous methods while also being more effective at producing certain types of chemical compounds that could potentially replace existing ones like phosgene.
Problems solved by technology
This patented technical problem addressed in this patents relates to improving the performance of certain chemical compounds that use specific ring structures called heterocycles or other groups attached to them (such as nitrogen) due to their ability to participate effectively during various processes such as oxidation/degrading redox reactions.
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
 Login to View More
Login to View More PUM
 Login to View More
Login to View More Abstract
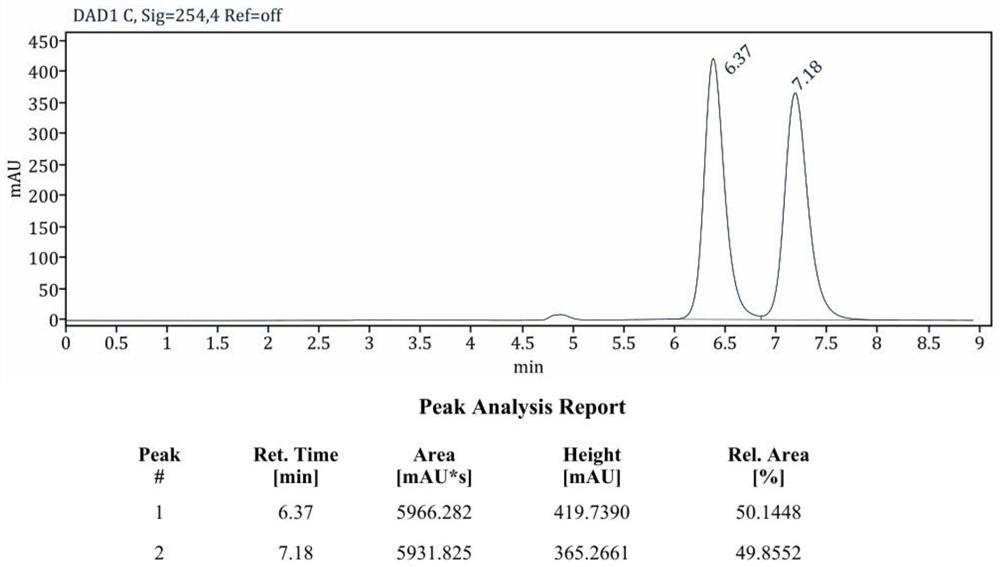
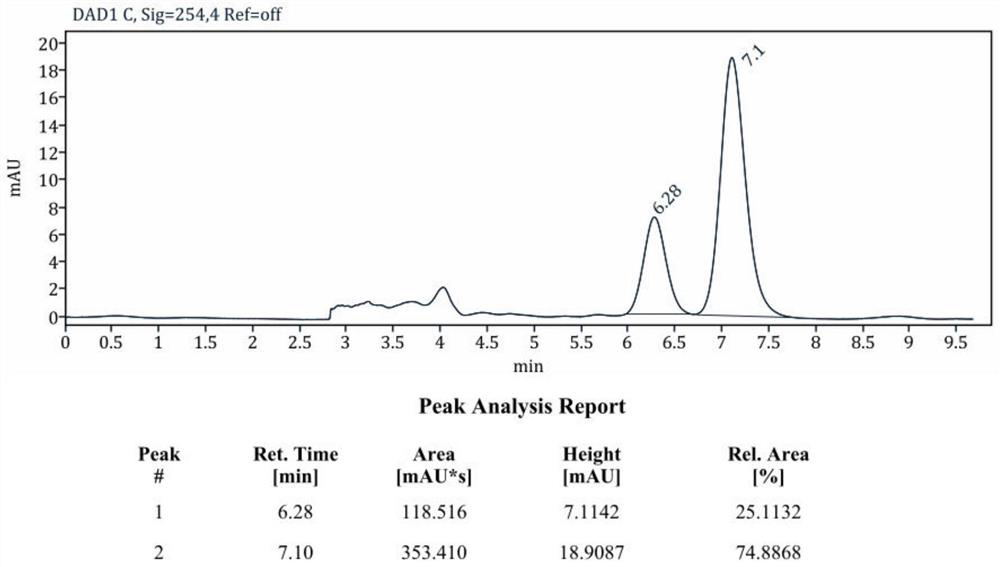
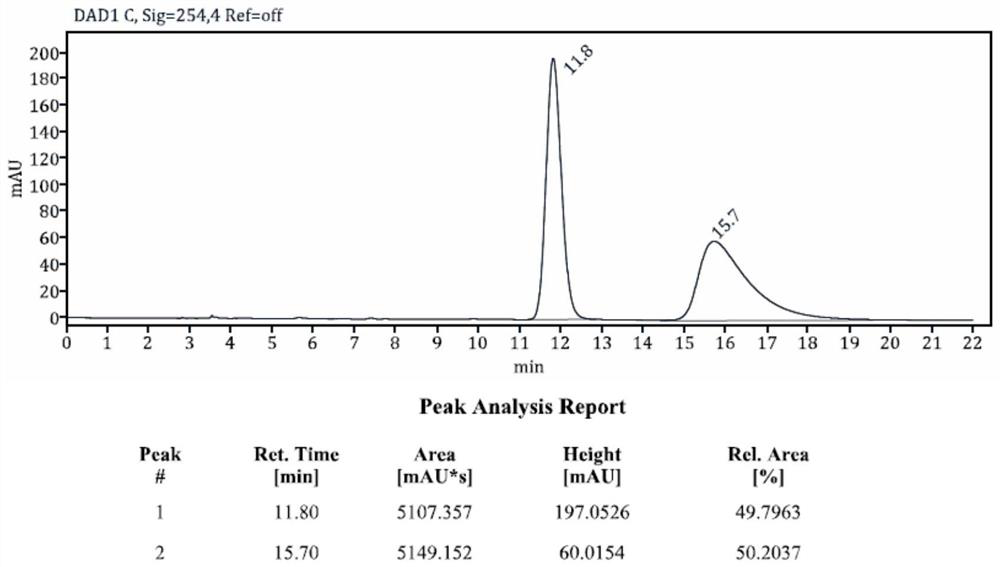
The invention discloses a chiral N-heterocyclic carbene catalyst as well as a preparation method and application thereof, and belongs to the technical field of organic chemistry. The preparation method of the chiral N-heterocyclic carbene catalyst specifically comprises the following steps: adding N-bromo-succinimide and p-toluenesulfonic acid into a round-bottom flask, dissolving the N-bromo-succinimide and p-toluenesulfonic acid with DCM, adding 1eq of cycloheptanone 1, and reacting at room temperature overnight; after the reaction is completed, concentrating and purifying the reactant to obtain alpha-bromo-cycloheptanone 2; then weighing 1g of montmorillonite, 10mmol of p-methylaniline and 40mmol of phenylacetylene, carrying out solvent-free reaction at 140 DEG C for 8 hours, cooling the reaction product to room temperature, filtering the reaction product, washing the reaction product with diethyl ether, collecting filtrate, concentrating and purifying the filtrate to obtain a compound 5, carrying out asymmetric hydrogenation reduction on the compound 5, and carrying out multi-step reaction on the compound 5 and alpha-bromo-cycloheptanone 2 to synthesize a target compound 7. The method is high in operability, novel in synthetic route and high in yield. The method can be successfully applied to the amide far-end C (sp3)-H activation tandem cyclization reaction, realizes asymmetric catalysis, and has a wide market application prospect.
Description
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More Owner CHENGDU UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com