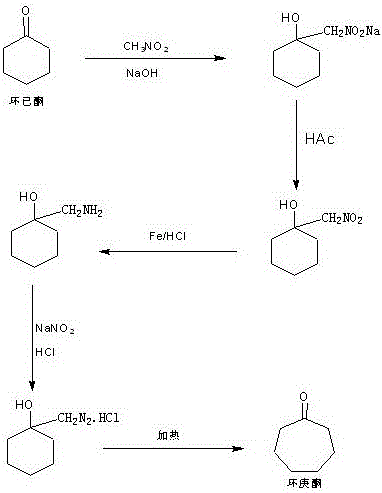
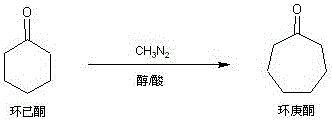
Method for preparing cycloheptanone from cyclohexanone through one step
A technology of cycloheptanone and cyclohexanone is applied in the field of preparation of intermediate cycloheptanone, can solve the problems of uneasy handling, long synthesis route and high production cost, and achieves the advantages of industrialized production, simple and convenient production operation and short synthesis route. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] A, prepare diazomethane ethanol saturated solution
[0019] In a 1000ml three-necked bottle, add 100g of 40% methylamine solution, after the system temperature drops to -5~0℃, slowly add 114g of 36% hydrochloric acid into the methylamine solution until the system pH=6~7, Then add 100g of urea, stir for about 10 minutes, then add a saturated sodium carbonate solution made of 2.6g of sodium carbonate mixed with 4ml of water, slowly raise the temperature to reflux, and keep the reflux reaction for 4 to 6 hours. After the reaction, the pH is detected to be about 7 -8, then lower the temperature of the system to 20-25°C, add 50ml of tap water, then add dilute sulfuric acid made of 82.5g of concentrated sulfuric acid and 225ml of tap water and cool to 5-10°C, and then further reduce it to -5-0 ℃, keep it at 0℃, slowly add the pre-prepared sodium nitrite solution made of 95g sodium nitrite mixed with 155ml tap water and cool it down, drop it in about 1 to 1.5 hours, after the ...
Embodiment 2
[0025] A. Preparation of methanol saturated solution of diazomethane
[0026] In a 1000ml three-necked bottle, add 100g of 40% methylamine solution, and after the system temperature drops to -5~0°C, slowly add 114g of 36% hydrochloric acid into the methylamine solution until the system pH=6-7, then Add 100g of urea, stir for about 10 minutes, then add a saturated sodium carbonate solution made of 2.6g of sodium carbonate mixed with 4ml of water, slowly raise the temperature to reflux, and keep the reflux reaction for 4-6 hours. After the reaction, the pH is 7- 8. Then lower the temperature of the system to 20-25°C, add 50ml of tap water, then add dilute sulfuric acid made of 82.5g of concentrated sulfuric acid and 225ml of tap water and cool to 5-10°C, and then further drop to -5~0°C , keep at about 0°C, slowly add the pre-prepared sodium nitrite solution prepared by adding 95g of sodium nitrite to 155ml of tap water and cool it down, drop it in about 1-1.5 hours, after the dr...
Embodiment 3
[0032] A, prepare diazomethane isopropanol saturated solution
[0033] In a 1000ml three-necked bottle, add 100g of 40% methylamine solution, and after the system temperature drops to -5~0°C, slowly add 114g of 36% hydrochloric acid into the methylamine solution until the system pH=6-7, Then add 100g of urea, stir for about 10 minutes, then add a saturated sodium carbonate solution made of 2.6g of sodium carbonate mixed with 4ml of water, slowly raise the temperature to reflux, and keep the reflux reaction for 4 to 6 hours. After the reaction, the pH is 7 ~8, then lower the temperature of the system to 20~25°C, add 50ml of tap water, then add dilute sulfuric acid made of 82.5g of concentrated sulfuric acid and 225ml of tap water and cool to 5~10°C, and then further reduce it to -5~0 ℃, keep it at about 0℃, slowly add the pre-prepared sodium nitrite solution prepared by adding 95g of sodium nitrite to 155ml of tap water and cool it down, and drop it in about 1 to 1.5 hours. St...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com