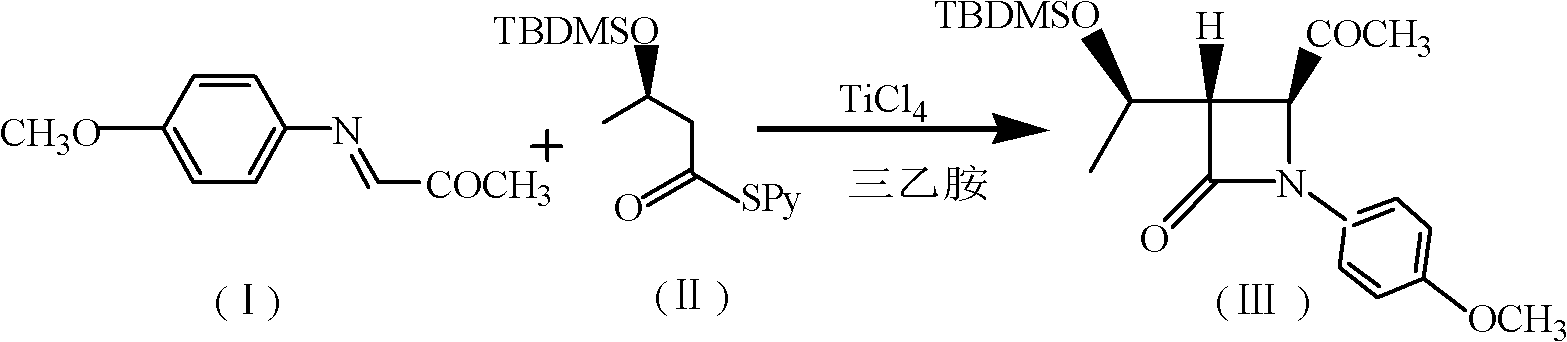Patents
Literature
52 results about "2-Azetidinone" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
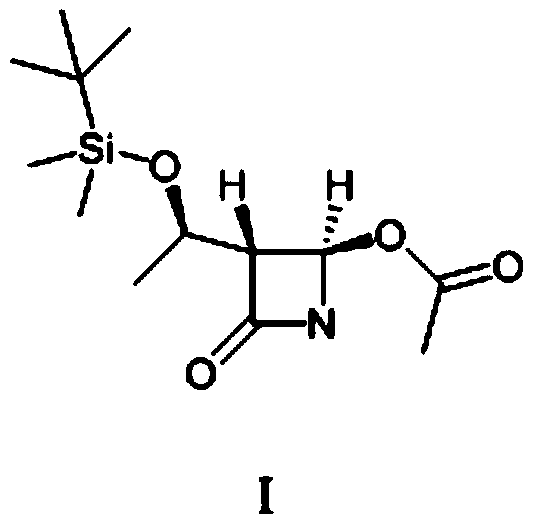
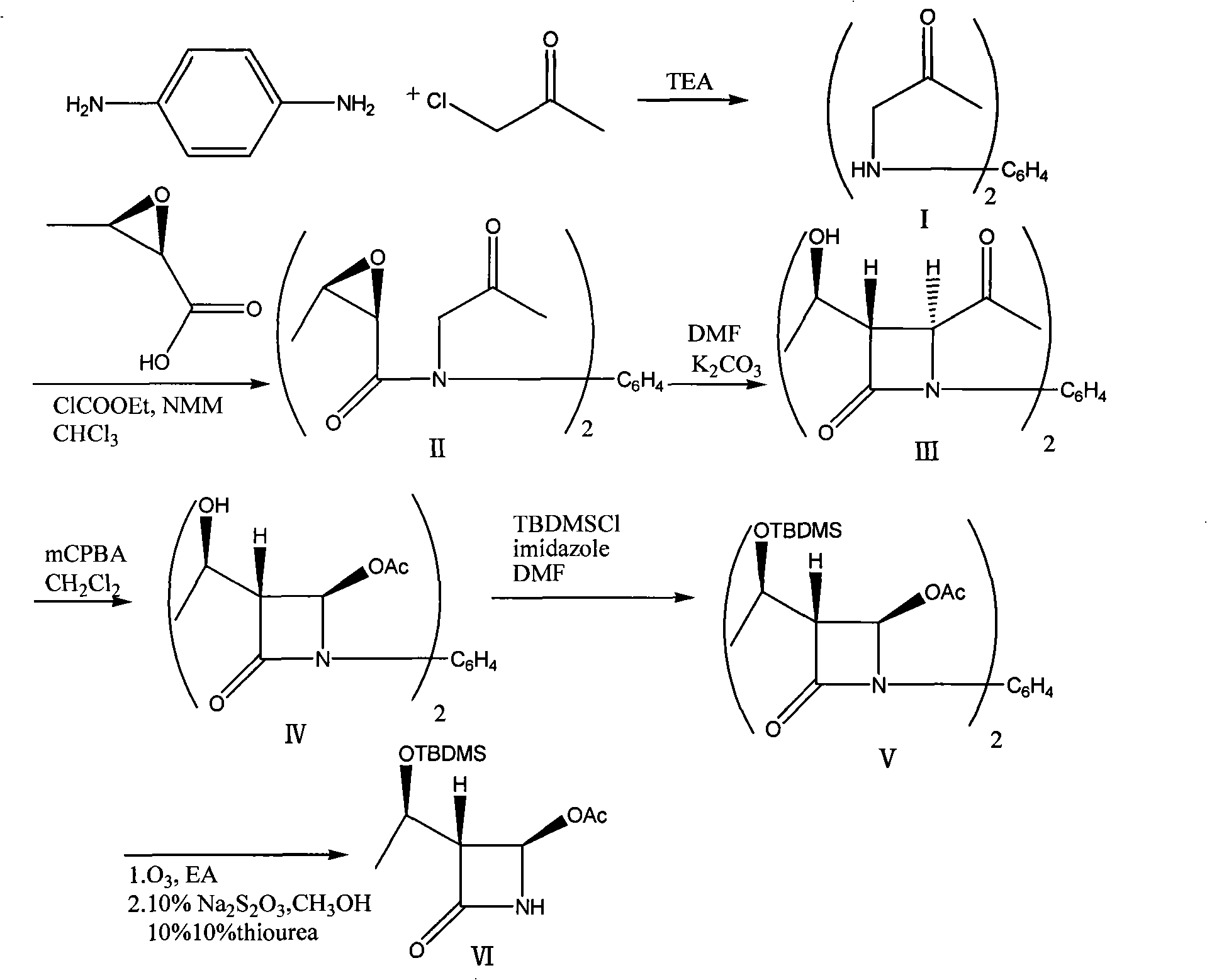
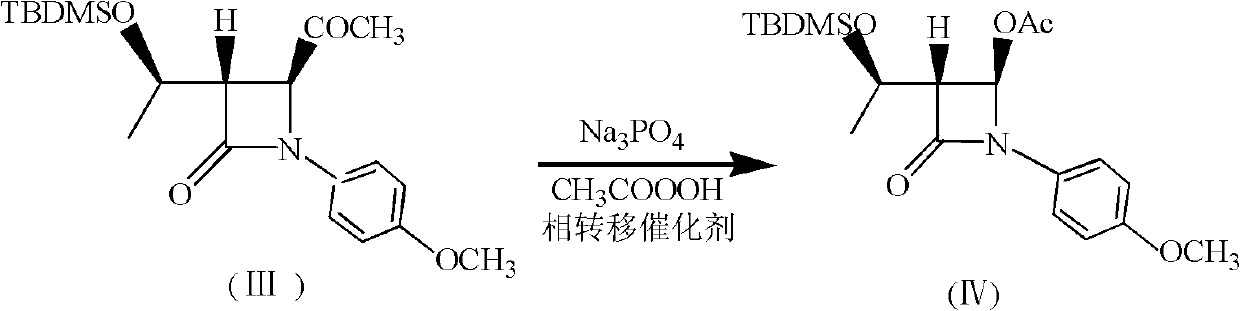
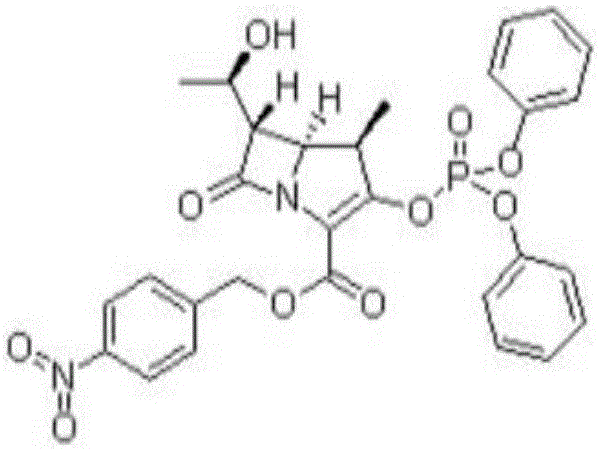
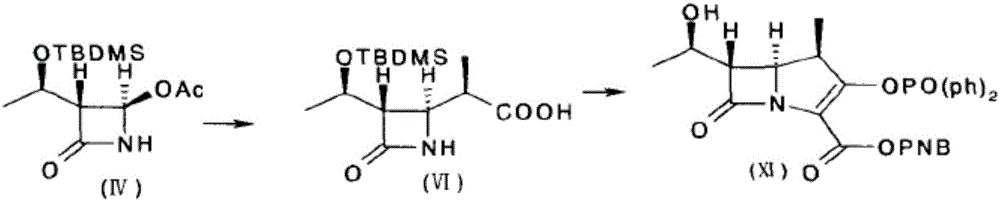
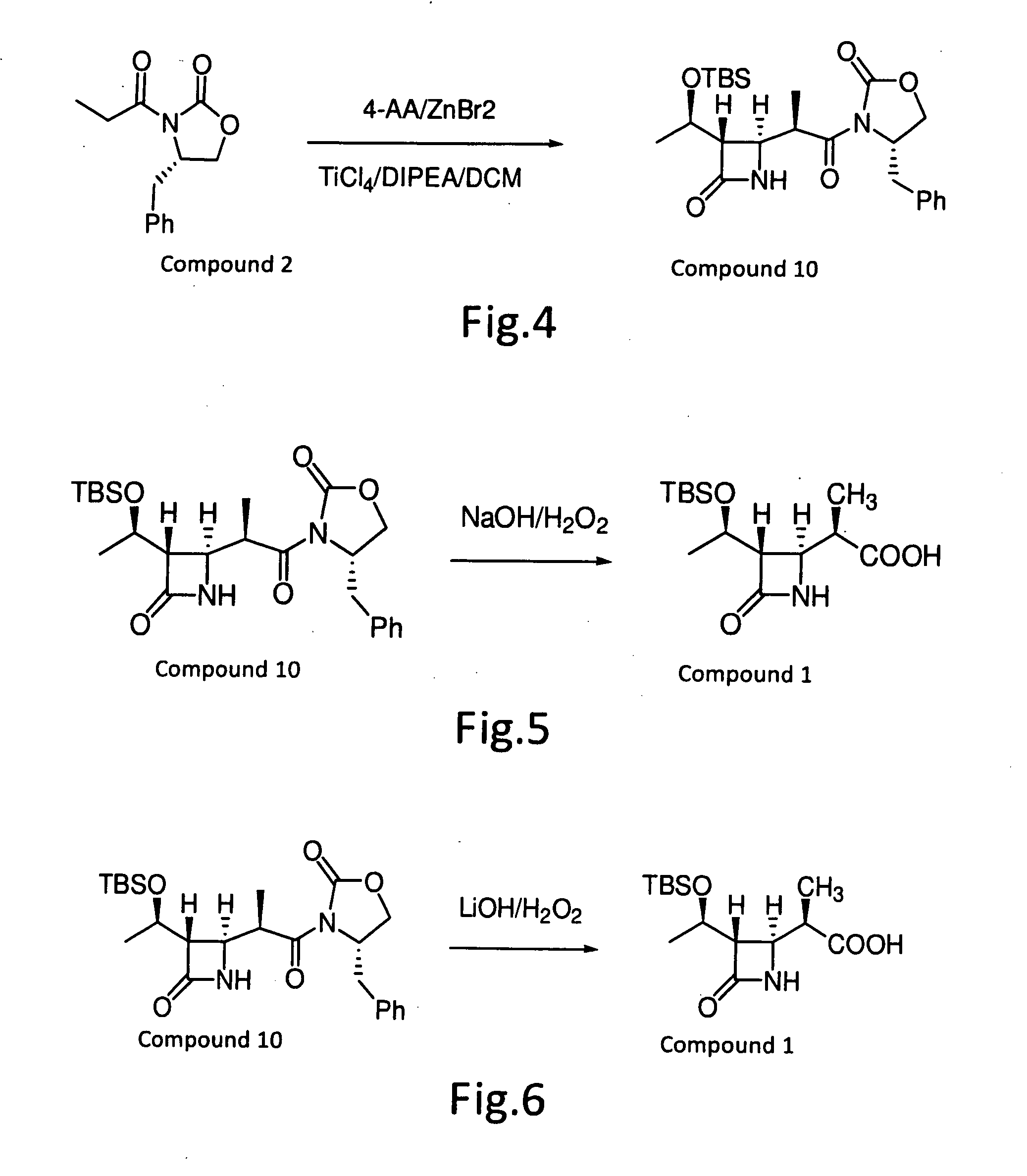
Method for preparing (3R,4R)-3-[(1R)tert-butyl-dimethyl-silyloxyethyl]-4-acetoxyl-2-azetidinone
InactiveCN102432632ASimple ingredientsMild responseGroup 4/14 element organic compoundsEthyl phosphate2-Azetidinone
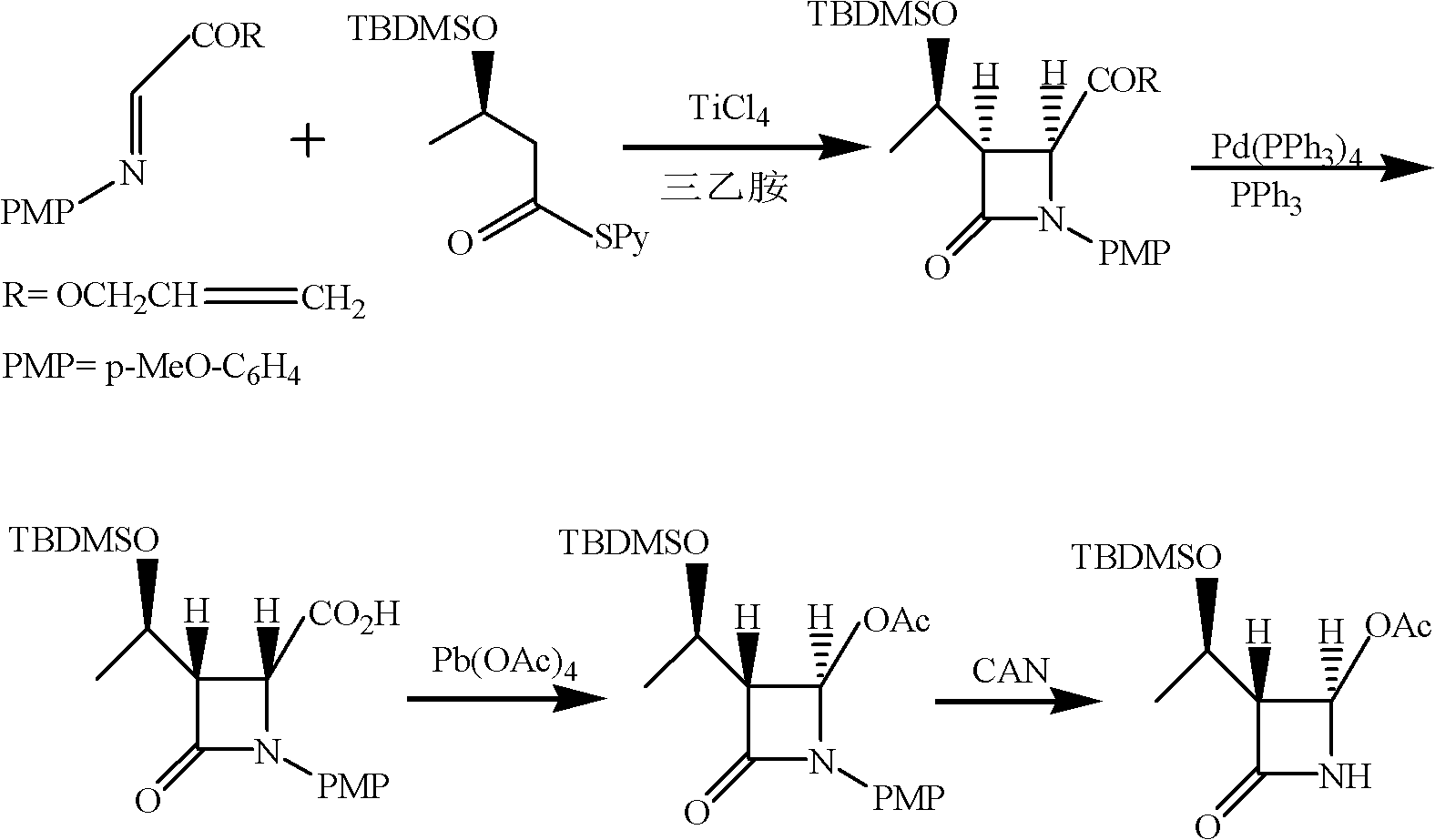
The invention relates to a method for preparing (3R,4R)-3-[(1R)tert-butyl-dimethyl-silyloxyethyl]-4-acetoxyl-2-azetidinone. The method comprises the following steps of: firstly, by using L-threonine as a raw material, preparing a key intermediate, namely (3S,4S)-1-para-methoxyphenyl-3-[(R)-1-hydroxyethyl]-4-acetyl-2-azetidinone by adopting a three-step one-pot method; secondly, introducing a silicon-based branched chain, and reacting acetyl into acetoxyl; and finally, removing methoxyphenyl to obtain a target product. The method is simple in raw material, mild in reaction, and environment-friendly, and facilitates industrial large-scale production.
Owner:SHANGHAI YUEANG CHEM
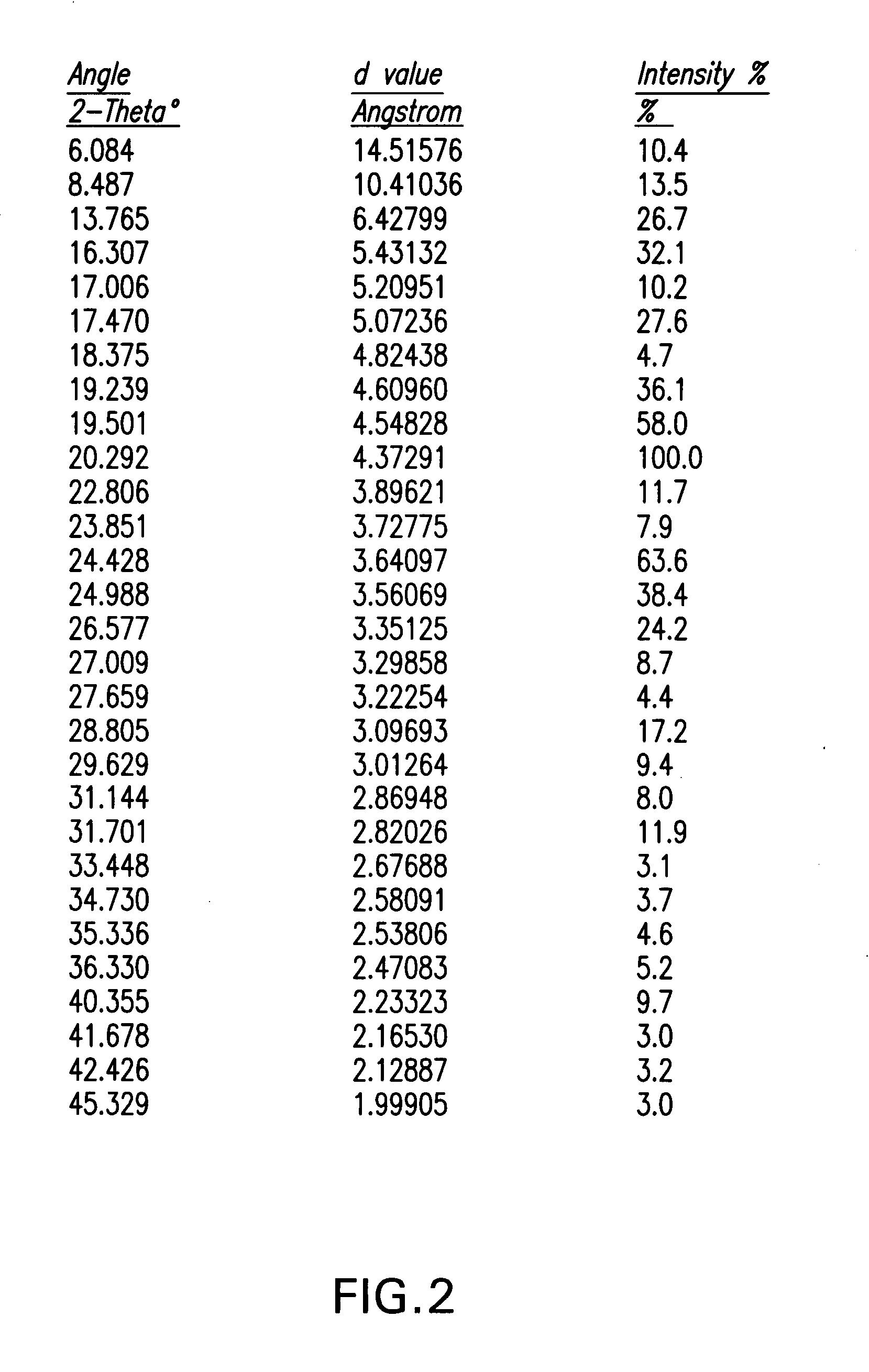
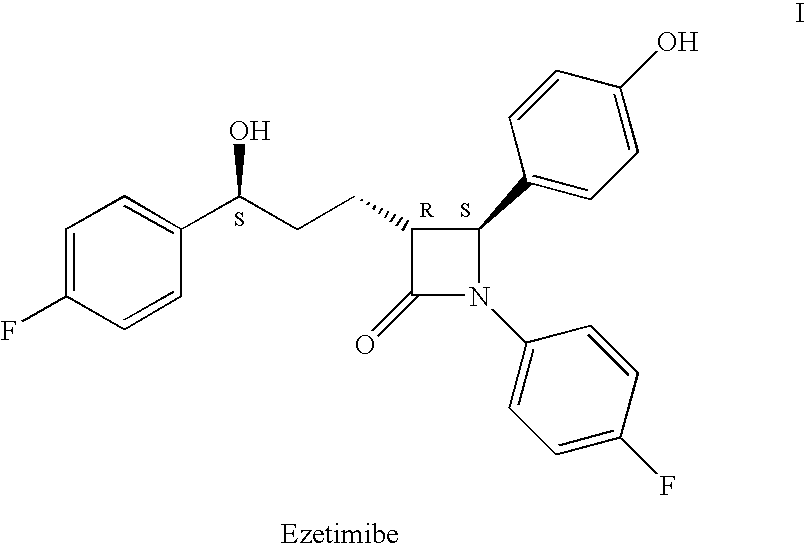
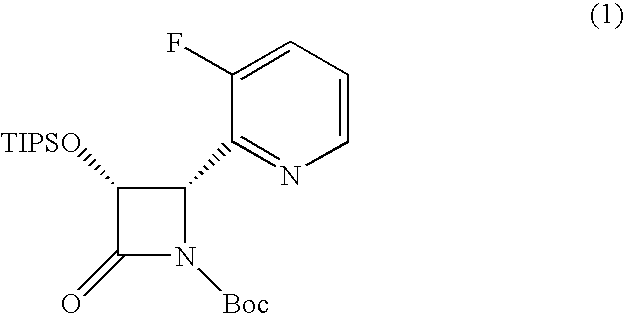
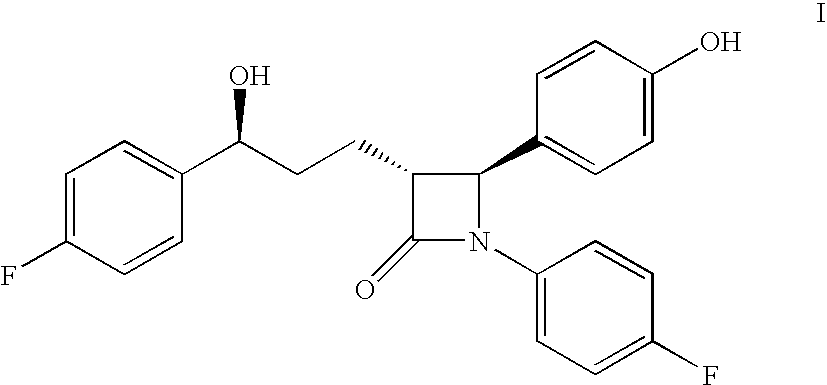
Processes for the preparation of (3R,4S)-4-((4-benzyloxy)phenyl)-1-(4-fluorophenyl)-3-((S)-3-(4-fluorophenyl)-3-hydroxypropyl)-2-azetidinone, an intermediate for the synthesis of ezetimibe
The invention encompasses (3R,4S)-4-((4-benzyloxy)phenyl)-1-(4-fluorophenyl)-3-(3-(4-fluorophenyl)-3-oxopropyl)-2-azetidinone (Compound 2a) having an enantiomeric purity of at least about 97.5%. The invention also encompasses Compound 2a having a chemical purity of at least about 97%. The invention further encompasses processes for preparing Compound 2a from Compound 1 having the following formula: The invention also encompasses processes for preparing a compound having the following formula: from a compound having the following formula: wherein R is selected from the group consisting of: H or a hydroxyl protecting group. The invention also encompasses processes for preparing Compound 2a, preferably to form Compound 2a-Form 01. Also included are processes for preparing ezetimibe from Compound 2a-Form 01 or Compound 2a prepared according to the invention, compositions containing such ezetimibe, and methods for reducing cholesterol using such compositions.
Owner:TEVA PHARM USA INC
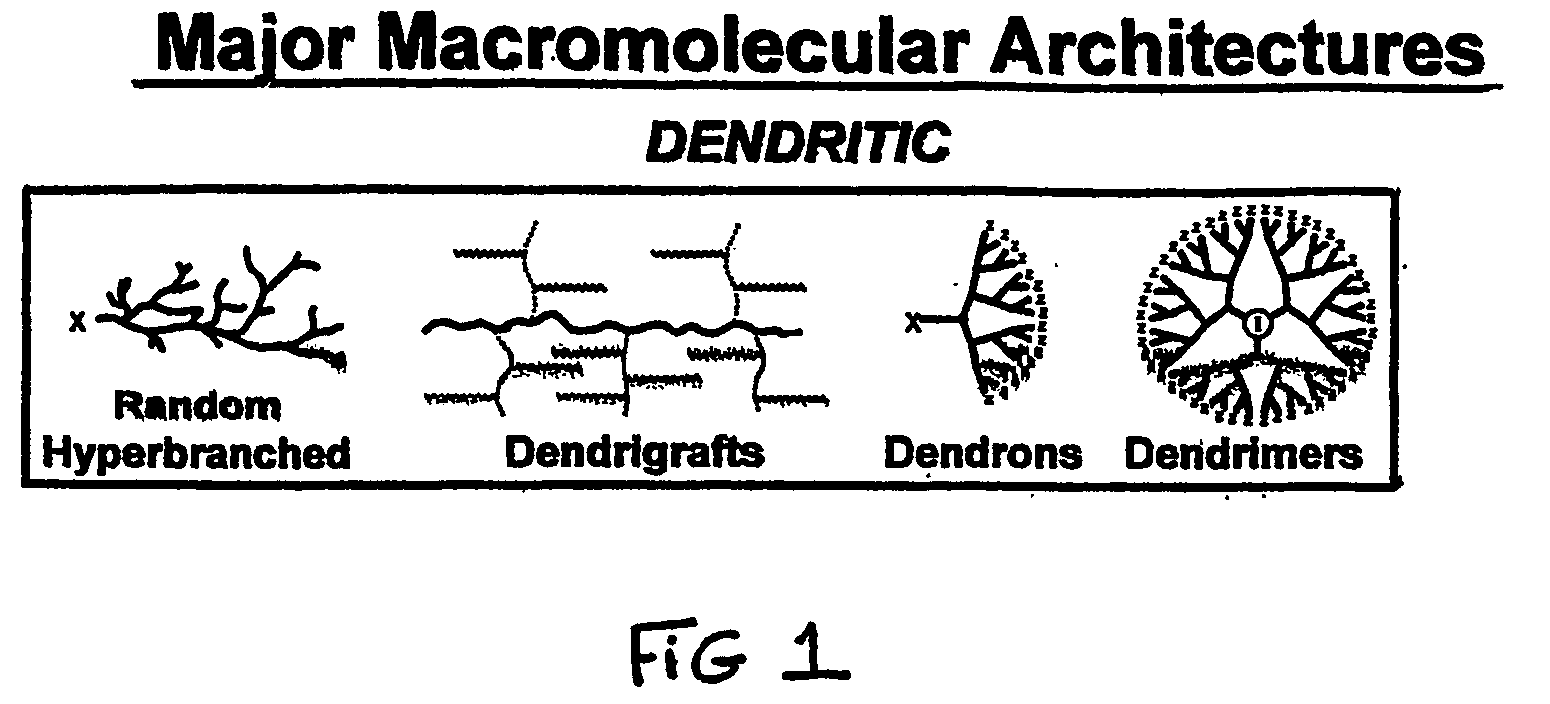
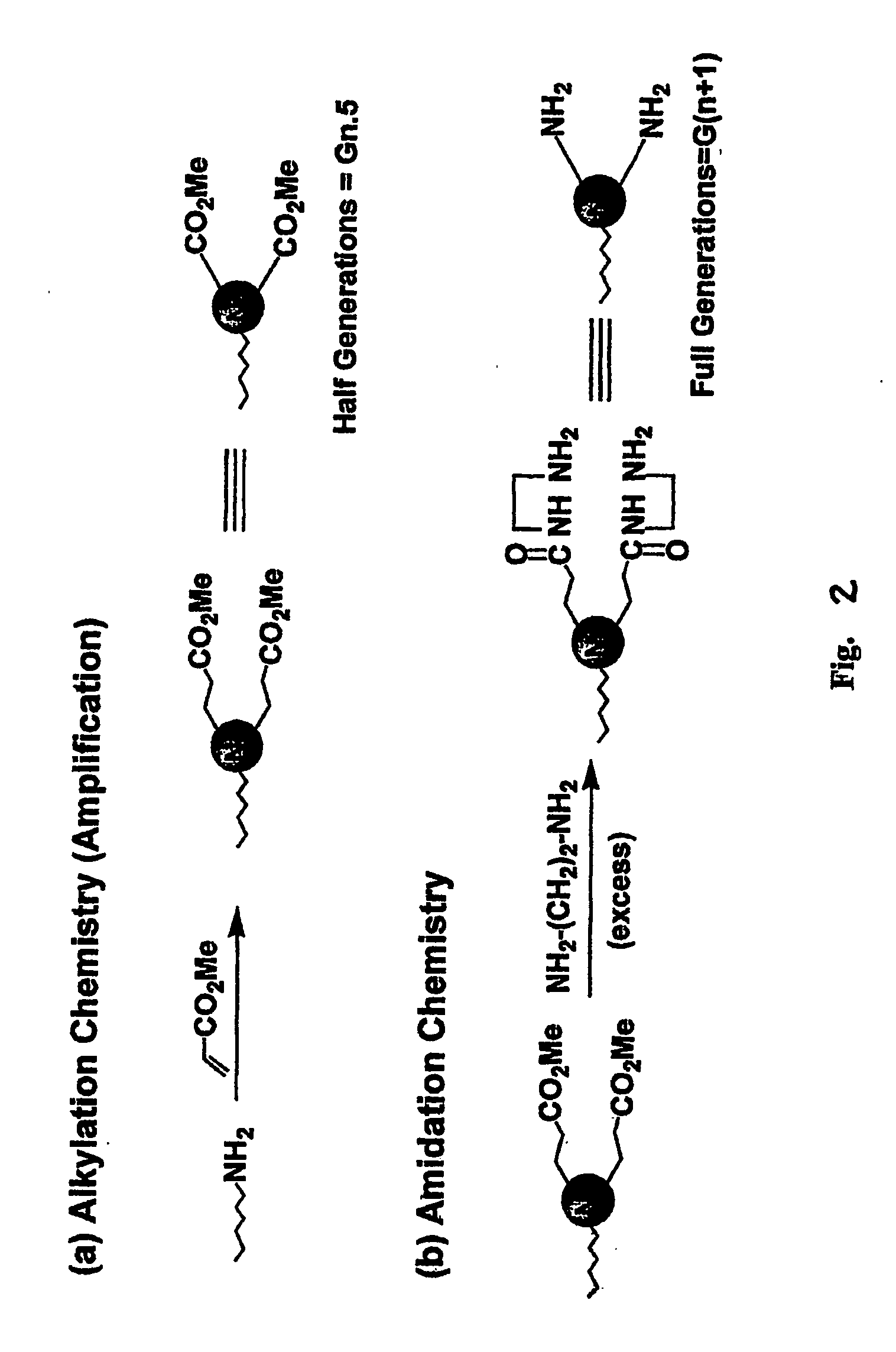
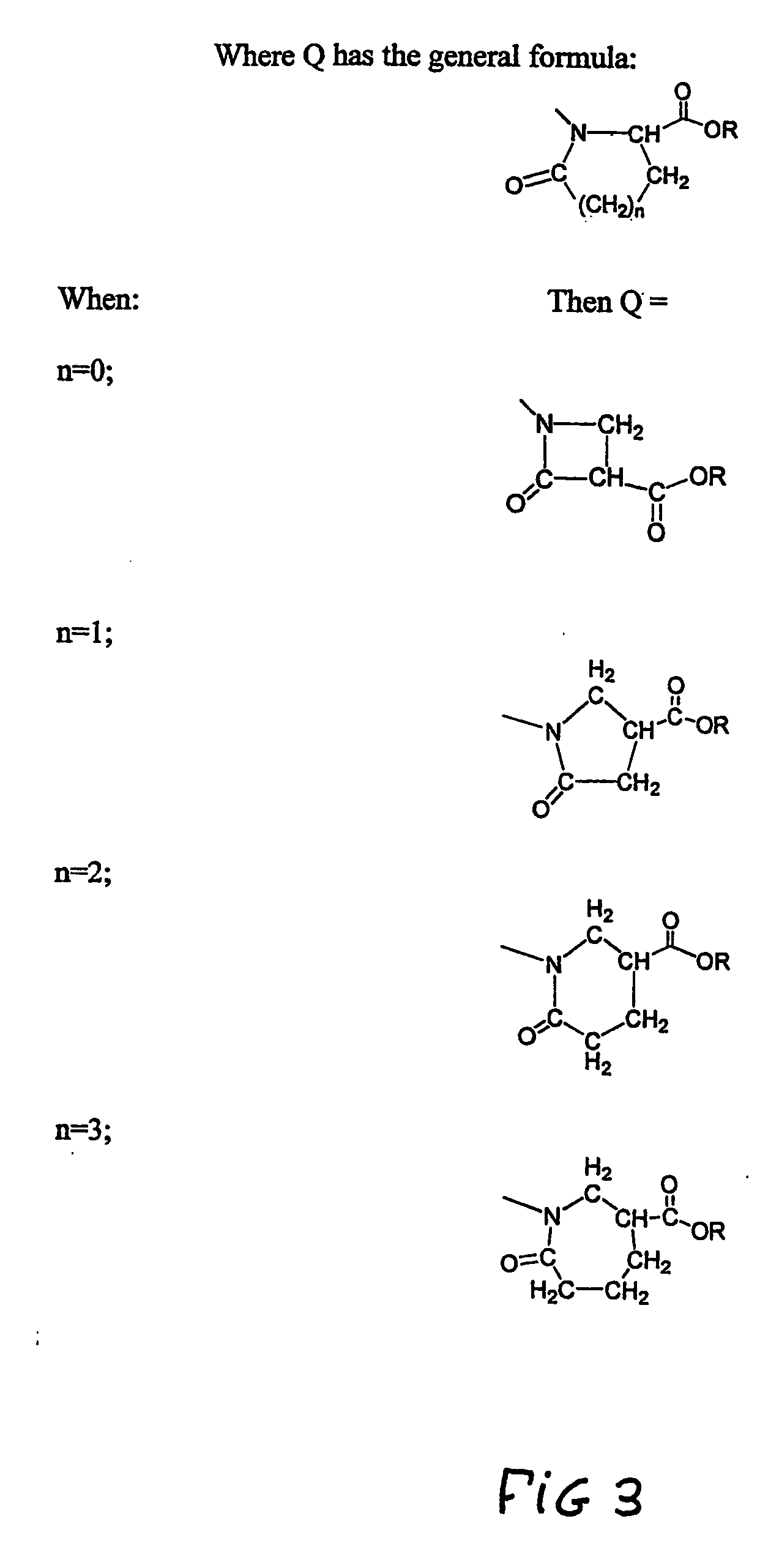
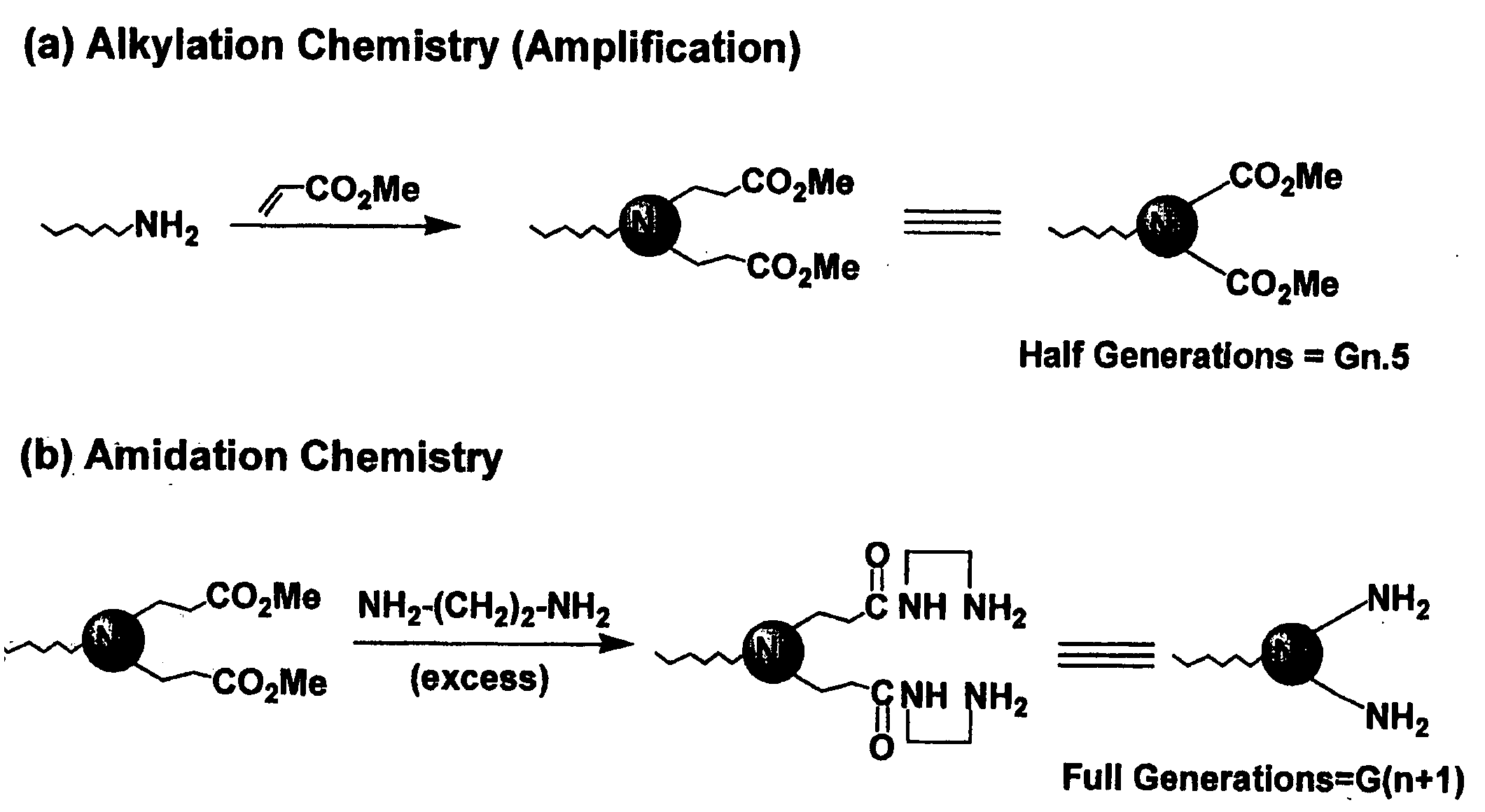
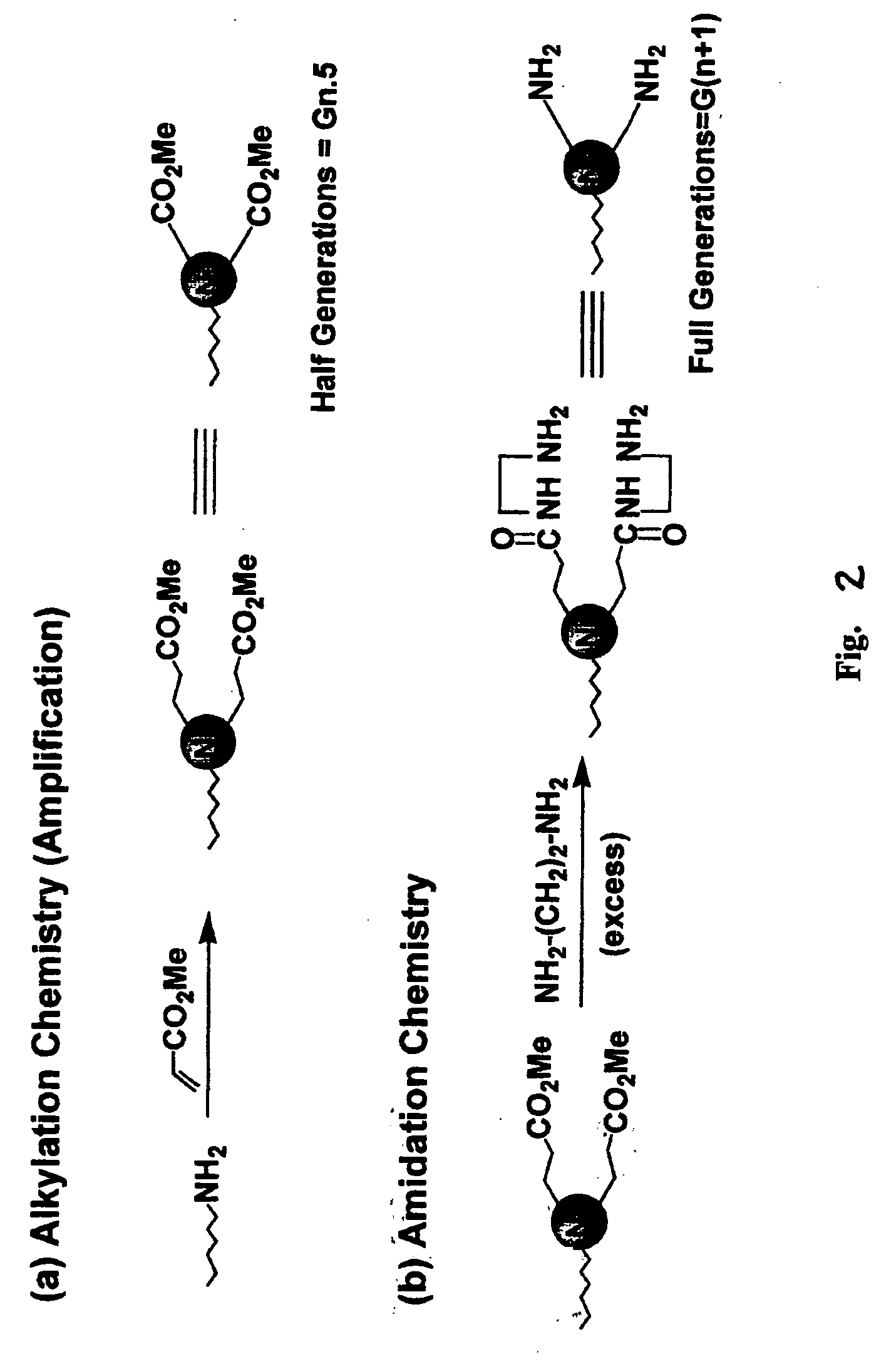
Heterocycle functionalized dendritic polymers
Heterocycle terminated dendritic polymers. More specifically, the production of 2-pyrrolidone, 2-piperidone, 2-aza-cycloheptanone or 2-azetidinone-terminated dendritic polymers obtained by reacting precursor primary amine, (e.g., —NH2)-terminated dendritic polymers with certain functionalized methacrylate reagents to produce new and novel dendritic polymers terminated with ester substituted 2-pyrrolidone, 2-piperidone, 2 aza-cycloheptanone or 2-azetidinone groups.
Owner:DENDRITIC NANO TECH INC
Process for the synthesis of azetidinone
InactiveUS20080032964A1Reduce cholesterolBiocideOrganic active ingredientsEzetimibeCombinatorial chemistry
Owner:TEVA PHARM USA INC
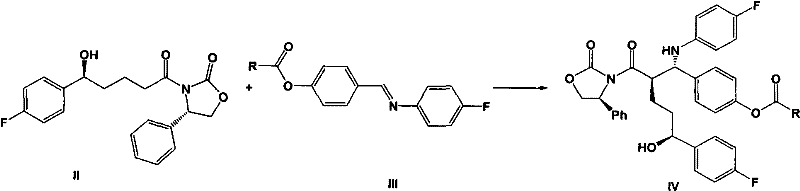
Novel ezetimibe synthesis method
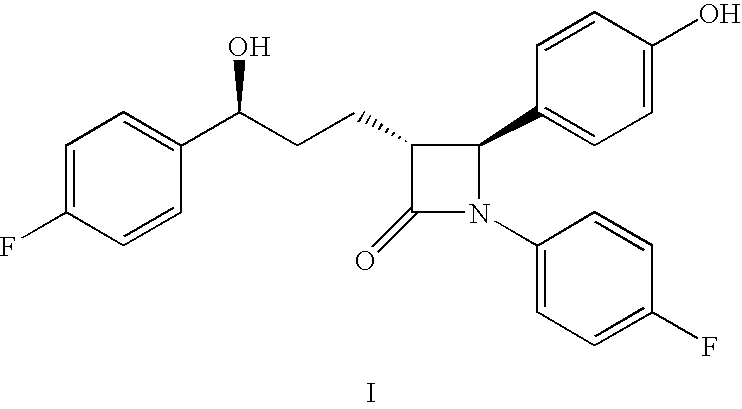
The invention discloses a novel ezetimibe synthesis method. The method comprises the following steps: reacting 3-[(5-(4-fluorophenyl)-(5S)-hydroxyvaleryl]-(4S)-phenyl-oxazolidinone (II) with N-4'-substituted acyloxy benzylidene-4-fluoroaniline (III) under the combined action of a tri-substituted chlorosilane and a titanium Lewis acid to generate 3-[(5-(4-fluorophenyl)-(5S)-hydroxyvaleryl]-3-{(2R)-[1-(4-substituted acyloxy phenyl)-(1S)-(4-fluoroanilino)]-(4S)-phenyl-oxazolidinone (IV), and sequentially treating the compound (IV) with BSA (bis(trimethylsilyl)acetamide), a fluoride ion cyclization catalyst and a proton acid solution to obtain a target product 1-(4-fluorophenyl)-(3R)-[3-(4-fluorophenyl)-(3S)-hydroxypropyl]-(4S)-(4-hydroxyphenyl)-2-azetidinone (I). The method has the advantages of low cost, simple operation, little environmental pollution, high product yield, high product purity and the like, and is especially suitable for industrial production.
Owner:ZHEJIANG HUAHAI PHARMACEUTICAL CO LTD +1
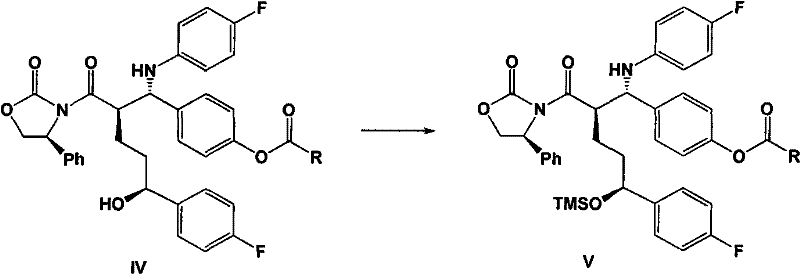
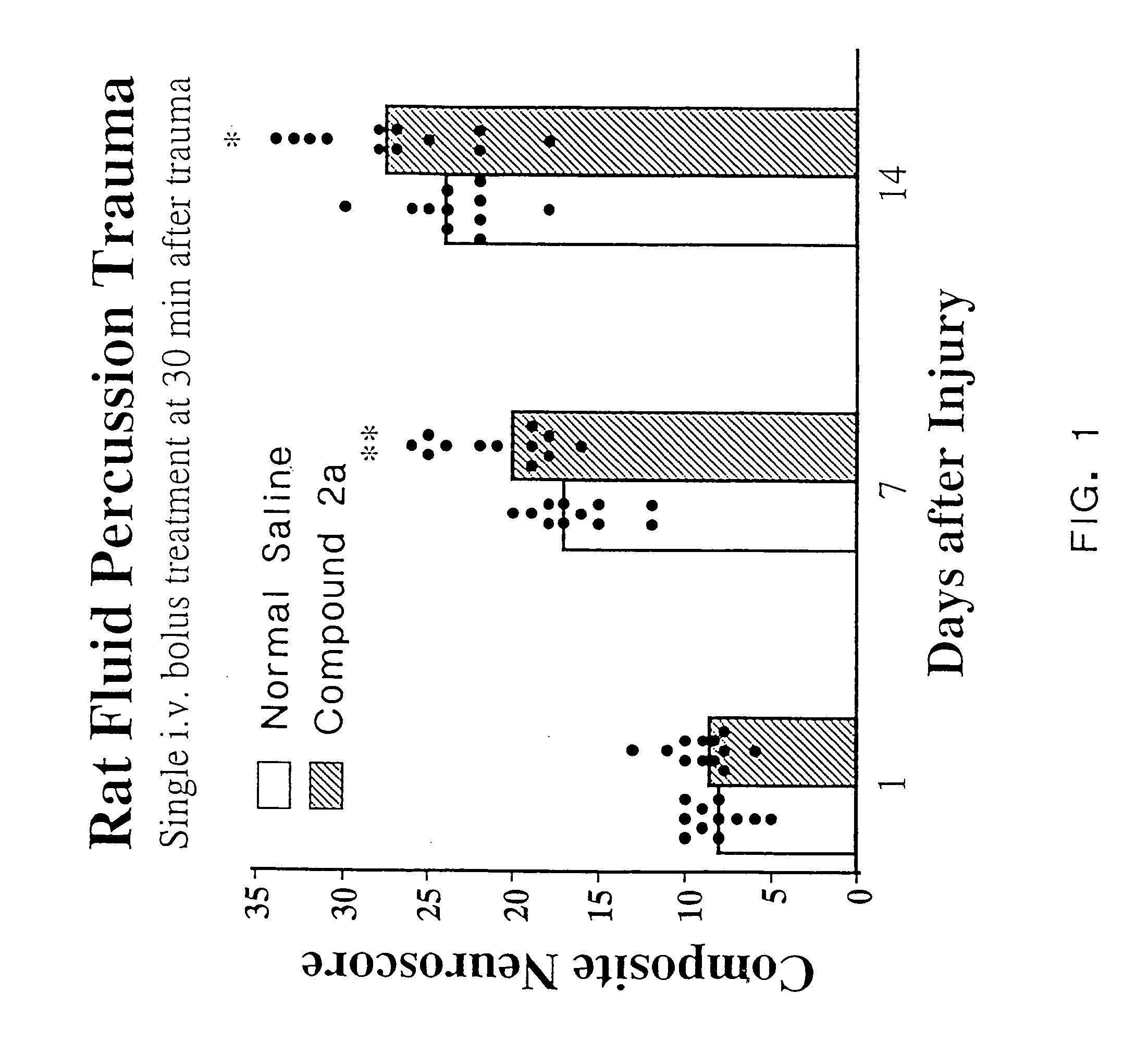
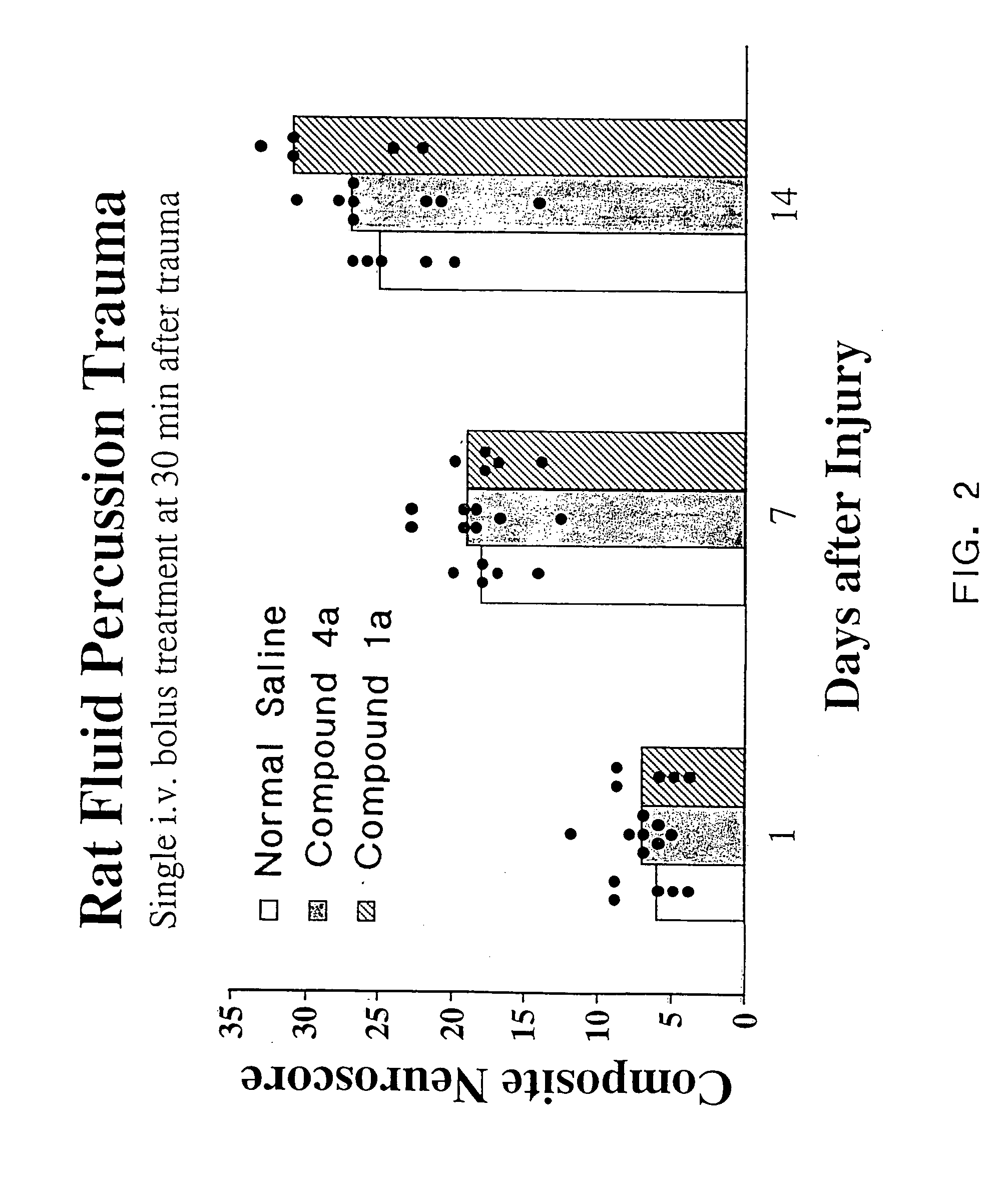
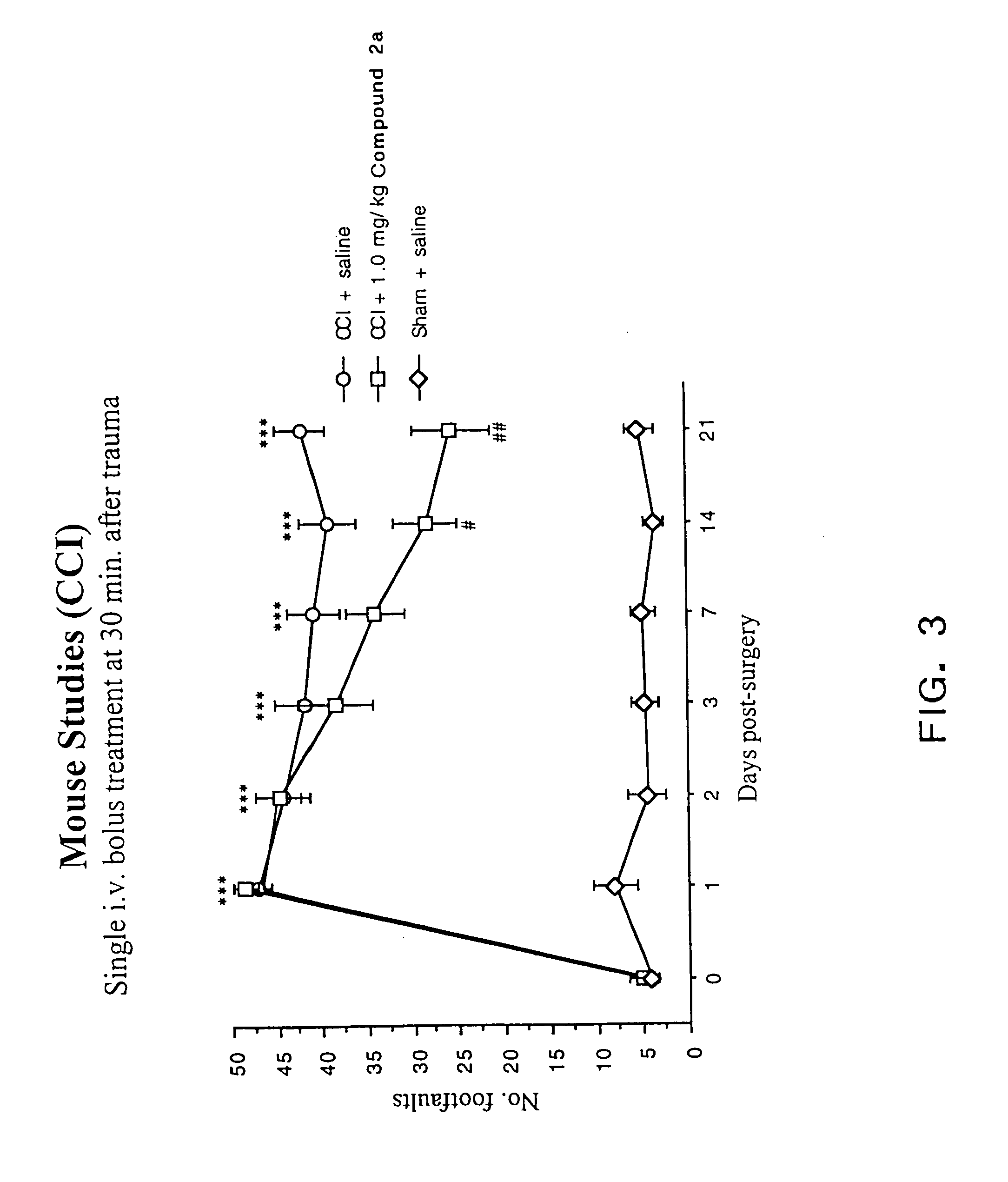
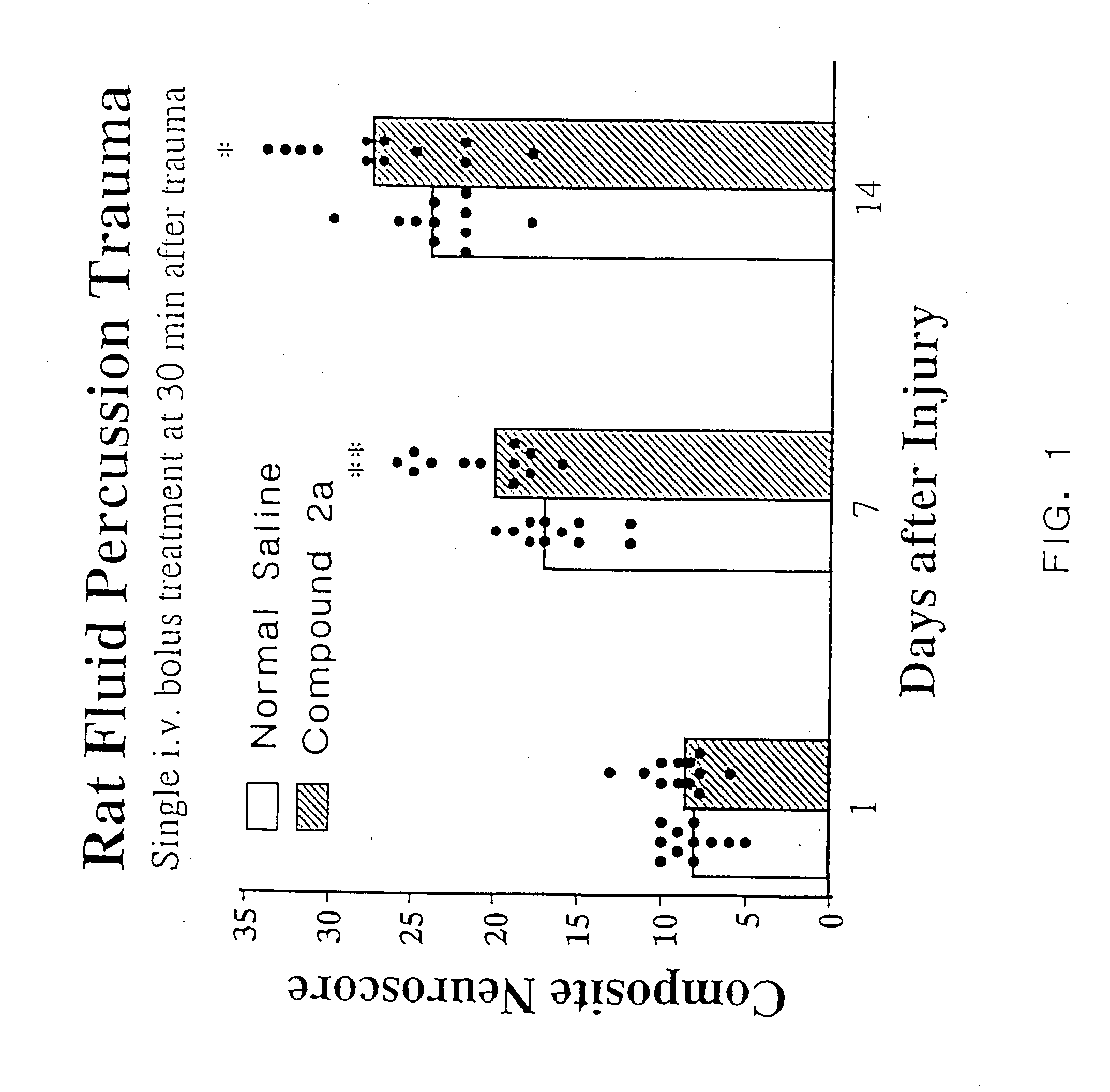
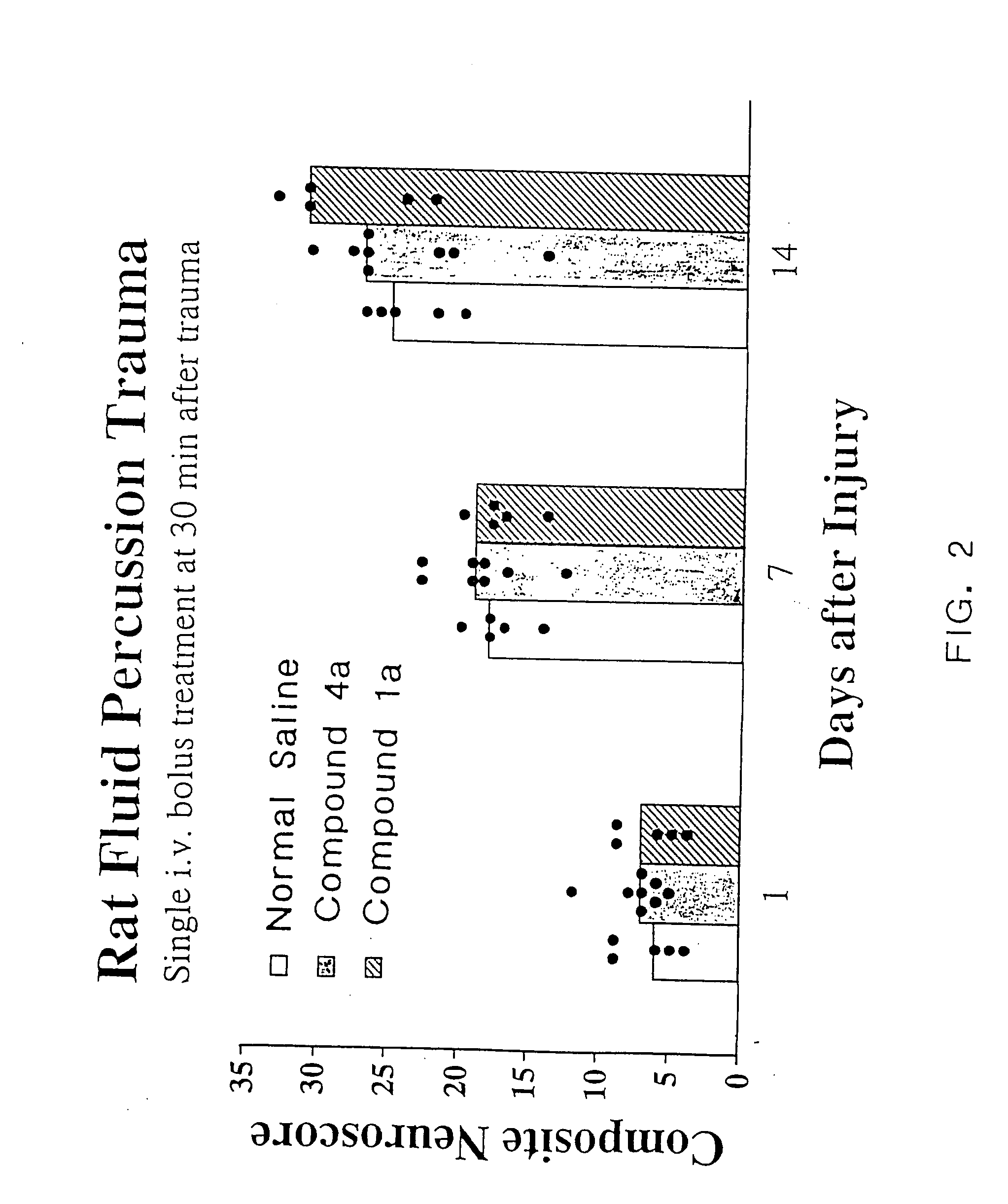
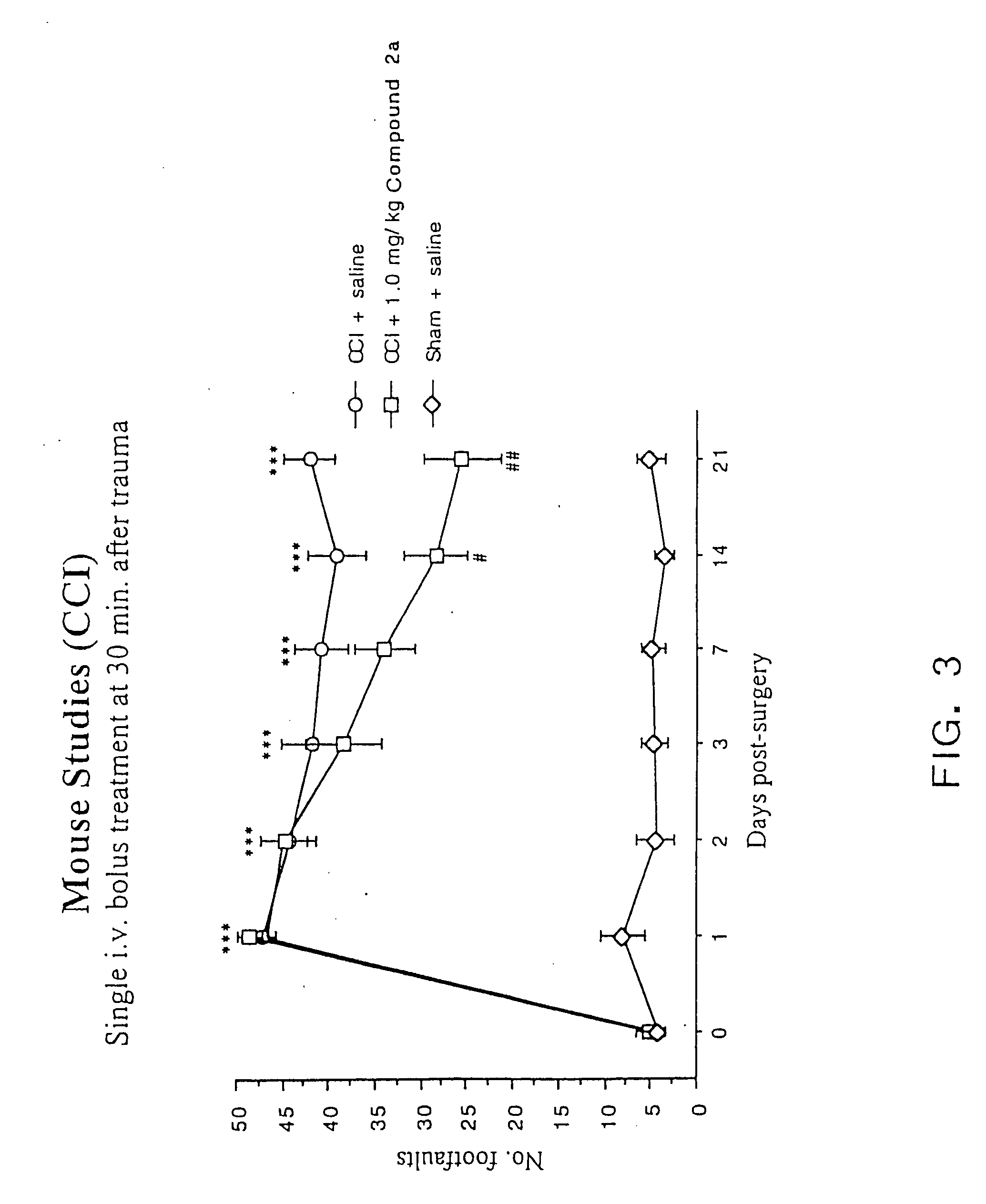
Cyclic dipeptides and azetidinone compounds and their use in treating CNS injury and neurodegenerative disorders
InactiveUS7202279B1Strong central nervous system (CNS) activityImprove cognitive functionBiocideNervous disorderDiseaseMedicine
The present invention provides 4-substituted-2-azetidinone compounds, bicyclic 2-5-diketopiperazine compounds, and pharmaceutical compositions thereof that are potent, safe and effective neuroprotective agents. Due to their strong central nervous system (CNS) activity, the compounds can be used to enhance memory and to treat a variety of neurological disorders. The compounds are particularly useful for treating neurological disorders caused by, or associated with, CNS trauma.
Owner:GEORGETOWN UNIV
Pyrrolidone, piperidone and azetidinone terminated and functionalizes dendritic polymers
InactiveUS20050171298A1Synthetic resin layered productsThin material handlingMethacrylate2-Pyrrolidone
Heterocycle terminated dendritic polymers. More specifically, the production of 2-pyrrolidone, 2-piperidone, 2-aza-cycloheptanone or 2-azetidinone-terminated dendritic polymers obtained by reacting precursor primary amine,(e.g., —NH2)-terminated dendritic polymers with certain functionalized methacrylate reagents to produce new and novel dendritic polymers terminated with ester substituted 2-pyrrolidone, 2-piperidone, 2-aza-cycloheptanone or 2-azetidinone groups.
Owner:DENDRITIC NANO TECH INC
Cyclic dipeptides and azetidinone compounds and their use in treating CNS injury and neurodegenerative disorders
InactiveUS20070161640A1Improve cognitive functionIncrease awarenessBiocideNervous disorderDiseaseMedicine
The present invention provides 4-substituted-2-azetidinone compounds, bicyclic 2-5-diketopiperazine compounds, and pharmaceutical compositions thereof that are potent, safe and effective neuroprotective agents. Due to their strong central nervous system (CNS) activity, the compounds can be used to enhance memory and to treat a variety of neurological disorders. The compounds are particularly useful for treating neurological disorders caused by, or associated with, CNS trauma.
Owner:GEORGETOWN UNIV
Process for synthesizing 4- acetoxy-2-azetidinone
ActiveCN101407486ASimple ingredientsMild reaction conditionsOrganic chemistryBulk chemical productionEpoxy4-acetoxy-2-azetidinone
The invention discloses a process for synthesizing 4-acetoxy-2-azetidinone. Epoxy butyrate is used as raw material with 1,4-2((oxo-propyl) amino) benzene to generate (2R, 3R)-1, 4-2((N-oxo-propyl-N-2, 3-epoxy butyl) amino) benzene which closes ring under the effect of potassium carbonate to get (3R, 4R)-1, 4-2((-3-((1'-R-hydroxide radical) ethide)-4-acetyl-2-azetidinone-1-radical) benzene of three chiral centers; benzoyl hydroperoxide is added to have a reaction to obtain (3R, 4R)-1, 4-2((-3-((1'-R-hydroxide radical) ethide)-4-acetoxy-2-azetidinone-1-radical) benzene; compound (3R, 4R)-1, 4-2((-3-((1'-R-tert-butyl dimethyl silica) ethide)-4-acetoxy-2-azetidinone-1-radical) benzene is obtained by tert-butyl dimethyl chlorosilane protection; and finally, (3R, 4R)-4-acetoxy-3-((1'-R-tert-butyl dimethyl silica) ethide)-2-azetidinone is obtained through ozone oxidation and protecting group removal. The method has simple raw material, moderate reaction condition, environmental friendliness,and can be applied to large scale industrialization production.
Owner:ZHEJIANG LEPU PHARMA CO LTD +1
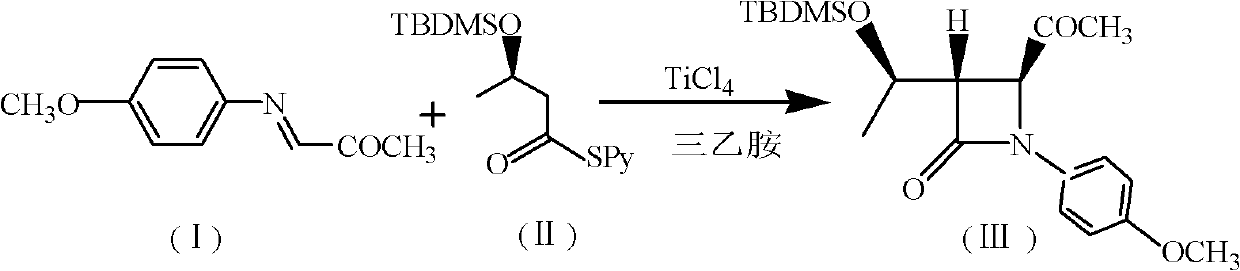
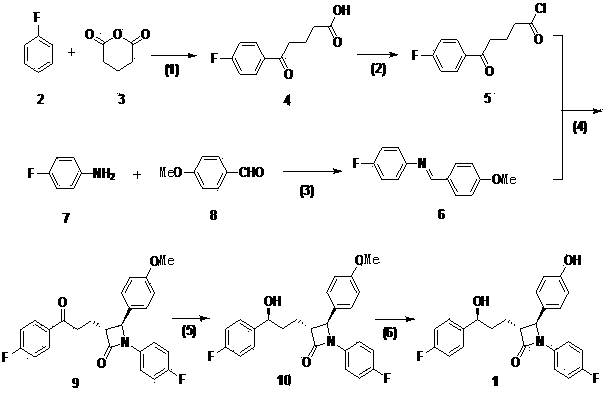
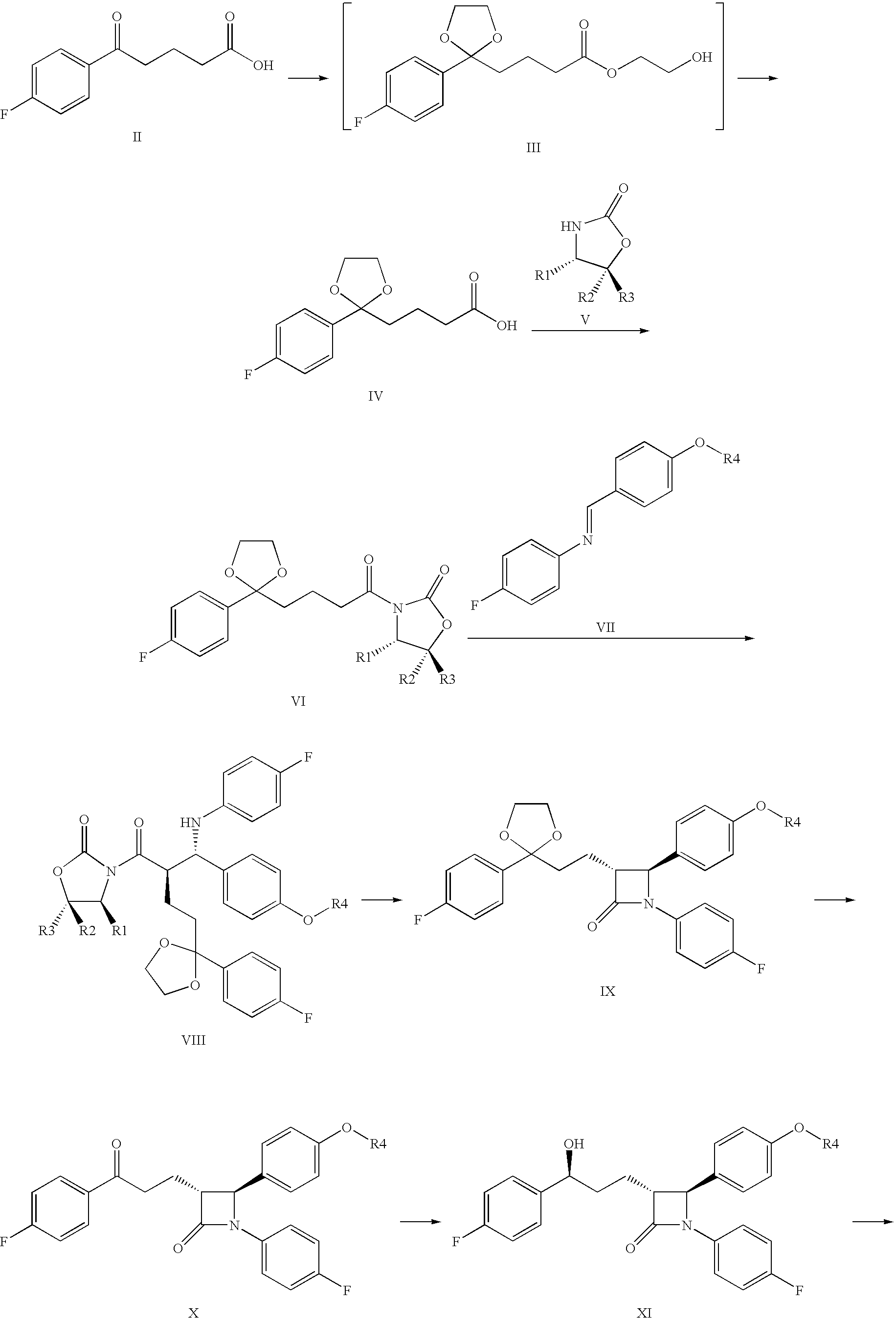
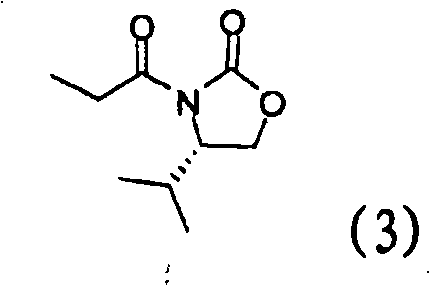
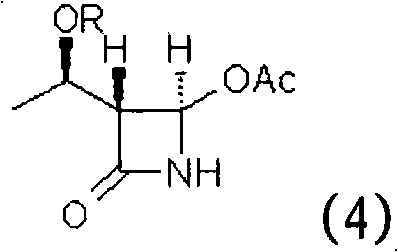
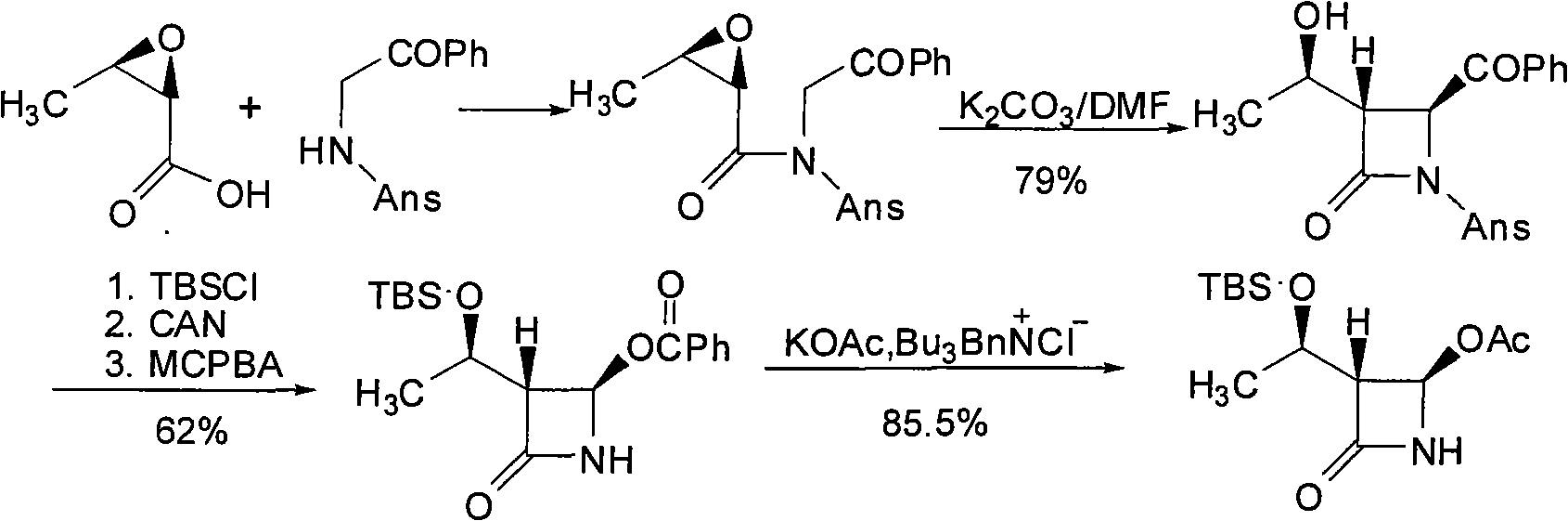
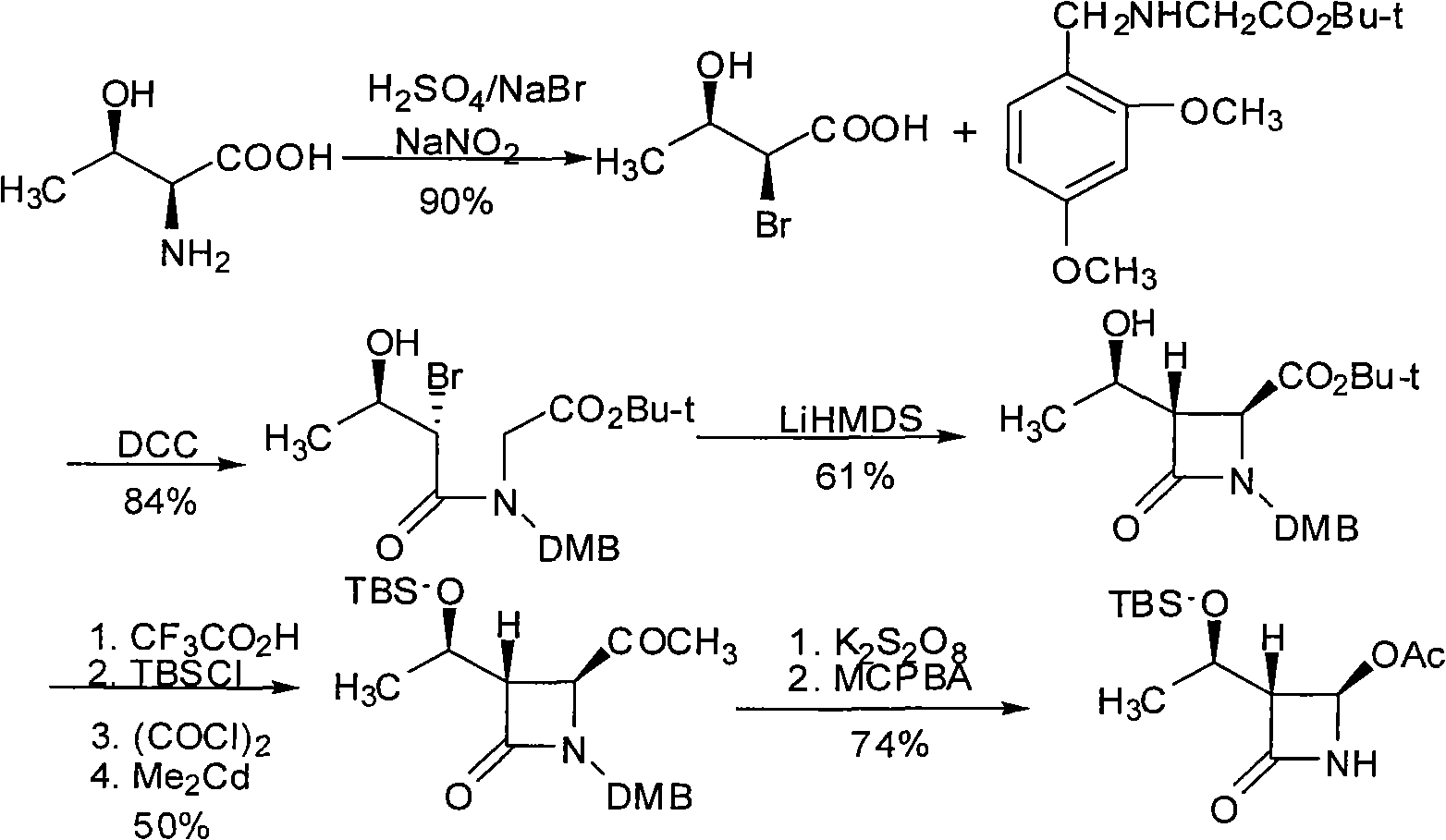
Intermediates for the preparation of (3r, 4s)-1-(4-fluorophenyl)-3-[(3S)-3-(4-fluorophenyl)-3-hydroxypropyl)]-4-(4-hydroxyphenyl)-2-azetidinone
InactiveUS20110046389A1Good diastereoselectivityHigh chemoselectivityOrganic chemistryBulk chemical productionKetoneSolvent
A method for the preparation of (S)-alcohol oxazolidides of general formula II, in which PG represents hydrogen or a hydroxyl protecting group, such as trimethylsilyl, tert-butyldimethylsilyl, benzyloxycarbonyl, tert-butoxycarbonyl, benzyl, benzhydryl or trityl, in which a ketal oxazolidide of general formula III, where PG has the same meaning as above and R means an alkyl with 1-4 carbon atoms, linear or branched, such as methyl, ethyl, isopropyl or butyl, or R+R together represents a divalent alkyl, or substituted with 1 or 2 alkyl groups, e.g. 1,2-ethylene, 1,2-propylene, 1,2-butylene, 1,3-propylene or 2,2-dimethyl-1,3-propylene, is deprotected by the action of acidic reagents in a mixture of water and a water-miscible solvent in the temperature range of 0 to 100° C. (stage A), and the obtained ketone oxazolidide of general IV, in which PG has the same meaning as above, is reduced with asymmetrical reagents in an inert organic solvent in the temperature range of −30 to +40° C. (stage B).
Owner:ZENTIVA AS
Synthesis method of 4-acetoxyl-2-azetidinone
ActiveCN102002066AReduce use costReduce pollutionGroup 4/14 element organic compoundsBulk chemical productionSynthesis methodsEconomic benefits
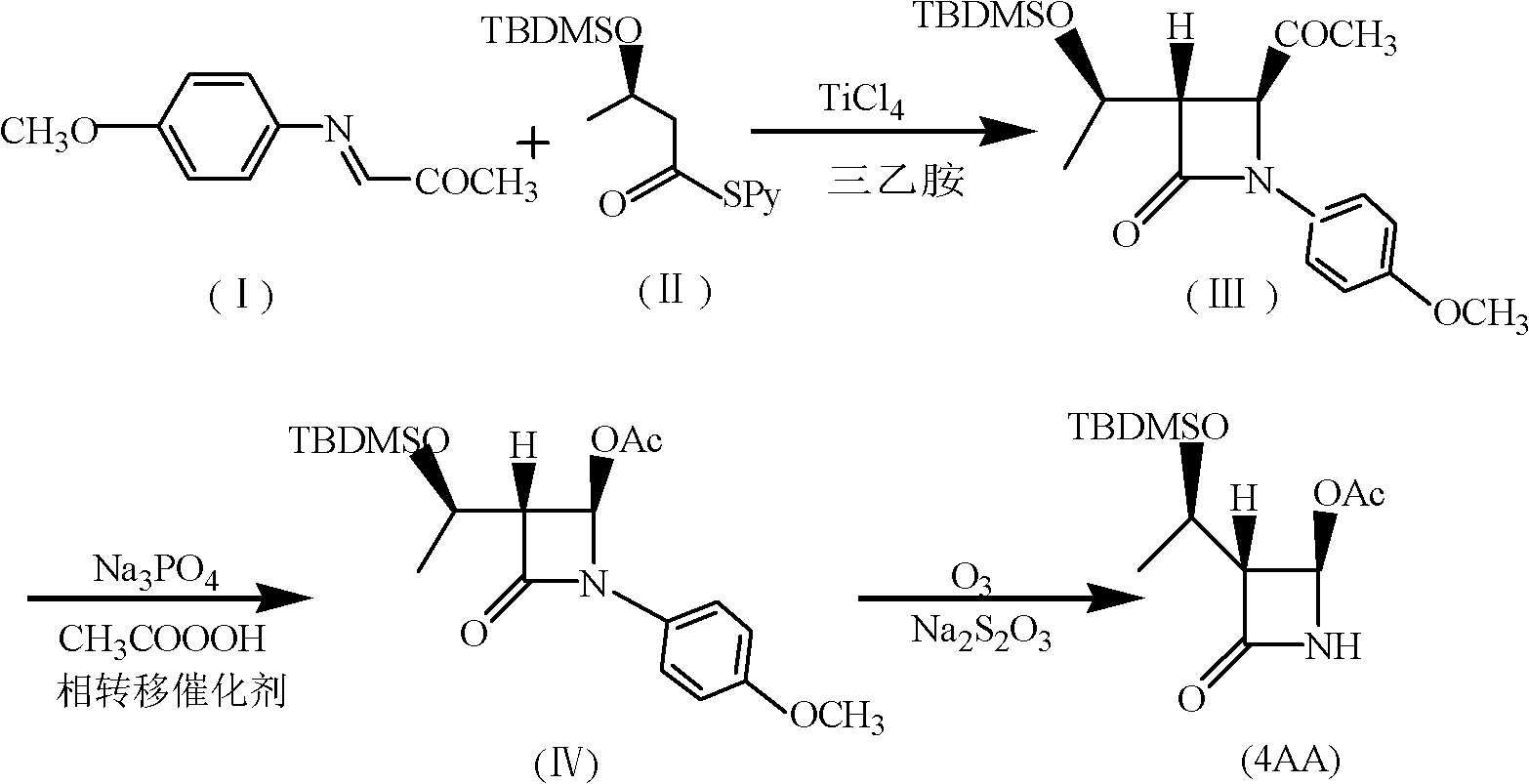
A synthesis method of 4-acetoxyl-2-azetidinone comprises the following steps of: (1) taking N-p-methoxypheny-N-(acetyl) methyleneimine as a raw material and carrying out cyclization reaction with (R)3-tert-butyldimethylsilyloxy-sulfo-butyrate-S-2-pyridinyl ester to obtain (3S,4S)-3-[(1'R)-tert-butyldimethylsilyloxyethyl]-4-acetyl-1-p-methoxyphenyl-2-azetidinone; (2) preparing (3R,4R)-3-[(1'R)-tert-butyldimethylsilyloxyethyl]-4-acetoxyl -1-p-methoxyphenyl-2-azetidinone from peroxyacetic acid through oxidization in the presence of Na3PO4 and a phase transfer catalyst; and (3) removing protective groups to obtain a final product (3R,4R)-3-[(1'R)-tert-butyldimethylsilyloxyethyl]-4-acetoxyl-2-azetidinone. The invention has the advantages of shortening reaction period, improving reaction total yield, and improving reaction yield and product purity by introducing the phase transfer catalyst in the step (2). The method is simple, causes small pollution and has great application value and economic benefit.
Owner:YIYUAN XINQUAN CHEM
Method for producing 2-azetidinone derivative
A process for producing a compound represented by the following formula (II) which comprises treating a compound represented by the following formula (I) (wherein R1, R2 and R3 represent each a specific substituent) with an enzyme capable of asymmetrically hydrolyzing an ester and the novel compound (II). The process of the present invention makes it possible to easily obtain an optically active 2-azetidinone derivative in a large amount at a low cost
Owner:DAIICHI SANKYO CO LTD
Method for synthesizing ezetimibe
The invention discloses a novel technical method for synthesizing ezetimibe (a compound 1), and belongs to the field of organic chemical medical synthesis. The method comprises the following steps: synthesizing a (3R, 4S)beta-lactam ring of a key intermediate (a compound 9) by taking p-anisaldehyde, 4-fluoroaniline and glutaric anhydride as main raw materials; and reducing and hydrolyzing the compound to obtain ezetimibe. The method disclosed by the invention adopts simply and easily-available materials, and is less in synthesis steps, and stable in process; a chiral four-membered ring of the key intermediate is directly constructed by virtue of one-step reaction to obtain an important intermediate (3R, 4S)-1-(4- fluorophenyl)-3-[(3S)-3-]4-fluorophenyl)-3-carbonyl propyl]-4-(4-methoxypheyl)-2-azetidinone (a compound 9); a target enantiomer proportion in the product is high, post-treatment operation is simple, and industrial large-scale production is easy to realize.
Owner:HENAN ACADEMY OF SCI CHEM RES INST CO LTD
Processes for the preparation of (3R,4S)-4-((4-benzyloxy)phenyl)-1-(4-fluorophenyl)-3-((S)-3-(4-fluorophenyl)-3-hydroxypropyl)-2-azetidinone, an intermediate for the synthesis of ezetimibe
The invention encompasses (3R,4S)-4-((4-benzyloxy)phenyl)-1-(4-fluorophenyl)-3-(3-(4-fluorophenyl)-3-oxopropyl)-2-azetidinone (Compound 2a) having an enantiomeric purity of at least about 97.5%. The invention also encompasses Compound 2a having a chemical purity of at least about 97%. The invention further encompasses processes for preparing Compound 2a from Compound 1 having the following formula:The invention also encompasses processes for preparing a compound having the following formula:from a compound having the following formula:wherein R is selected from the group consisting of: H or a hydroxyl protecting group. The invention also encompasses processes for preparing Compound 2a, preferably to form Compound 2a-Form 01. Also included are processes for preparing ezetimibe from Compound 2a-Form 01 or Compound 2a prepared according to the invention, compositions containing such ezetimibe, and methods for reducing cholesterol using such compositions
Owner:KANSAL VINOD KUMAR +3
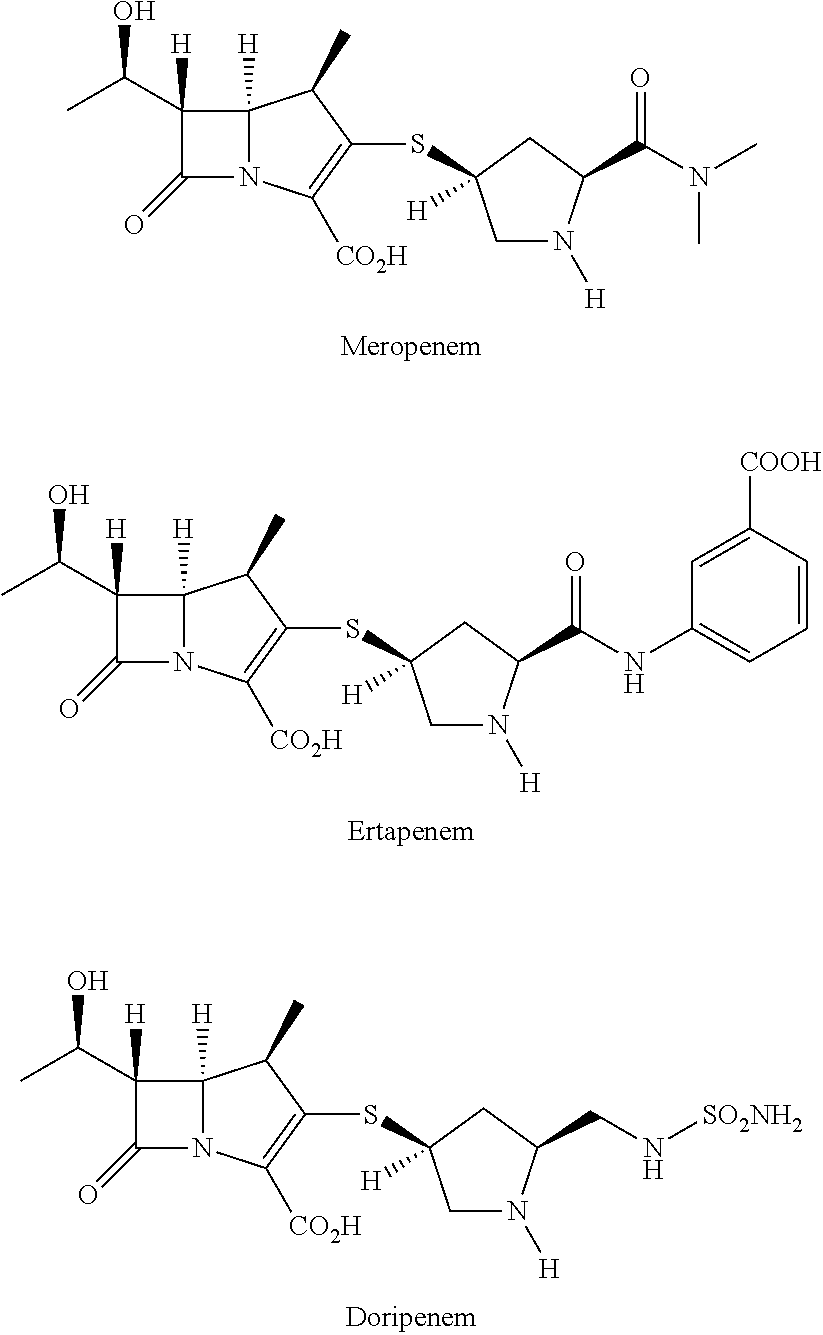
Preparation method for synthesizing key intermediate 4-BMA of 1beta-methyl carbapenem antibiotic bicyclic nucleus
The invention relates to a preparation method for synthesizing a key intermediate 4-BMA of 1beta-methyl carbapenem antibiotic bicyclic nucleus and belongs to the technical field of synthesis of carbapenem antibiotics. The method disclosed by the invention comprises the following steps: taking an aqueous solution of tetrahydrofuran as a solvent, taking indium as a catalyst, taking (3R,4R)4-carbethoxy-3-[(R)-((tert-butyldimethylsilyl)oxy)ethyl]-2-2-azetidinone (IV) as a raw material, and carrying out a reaction with alpha-bromo-acrylamide XV with large inductive groups so as to produce a compound XIV; not separating the reaction solution of the compound XIV, and performing oxidation-hydrolysis, thereby obtaining the target product VI. The method disclosed by the invention is stable in process, short in reaction route, simple and convenient in operation, mild in reaction conditions, high in product purity, few in three wastes, low in cost, high in yield and suitable for large-scale industrial production.
Owner:YIYUAN XINQUAN CHEM
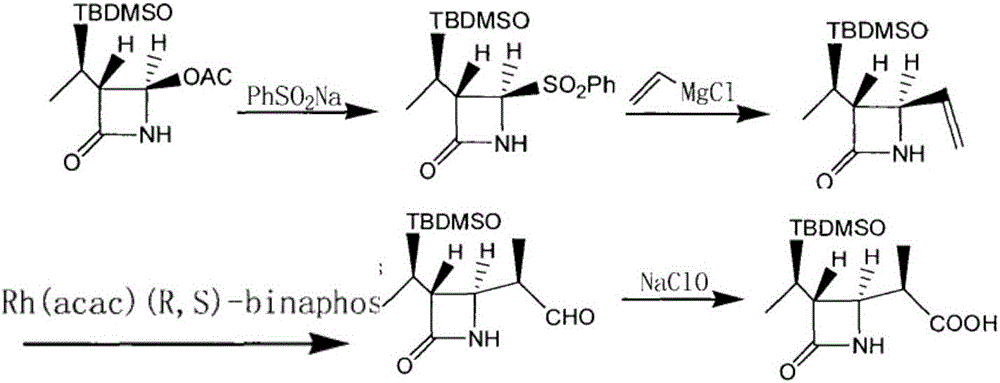
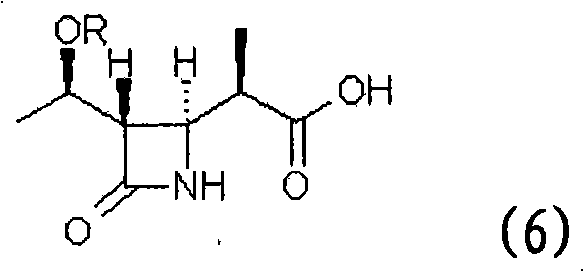
Method for manufacturing stereoselective preparation of 4-BMA using a chiral auxiliary and chiral auxiliary
The present invention relates to a process for preparing (3R,4S)-3-[[[R]-1′-t-butyldimethylsilyloxy]ethyl]-4-[(R)-1″-carboxyethyl]-2-azetidinone (beta-methylazetidin-2-one; 4-BMA), a key intermediate for the synthesis of carbapenem and penem antibiotics. Specifically, the present invention relates to a process comprising first, the preparation of a chiral auxiliary from cheap L-Phenylalaninol, and then the preparation of 4-BMA in high yield and high selectivity, under industrially mild condition.
Owner:SAVIOR LIFETEC CORP
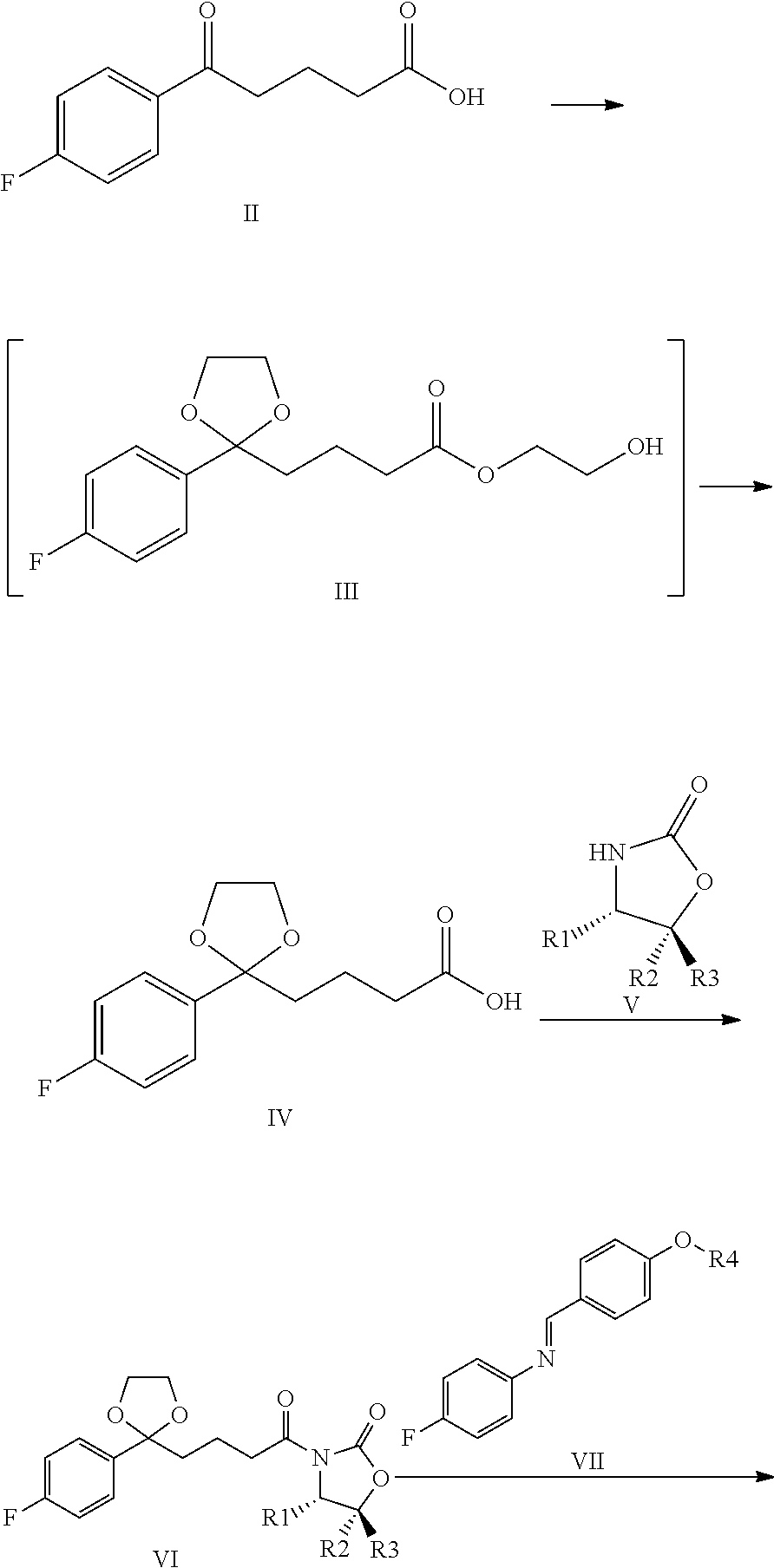
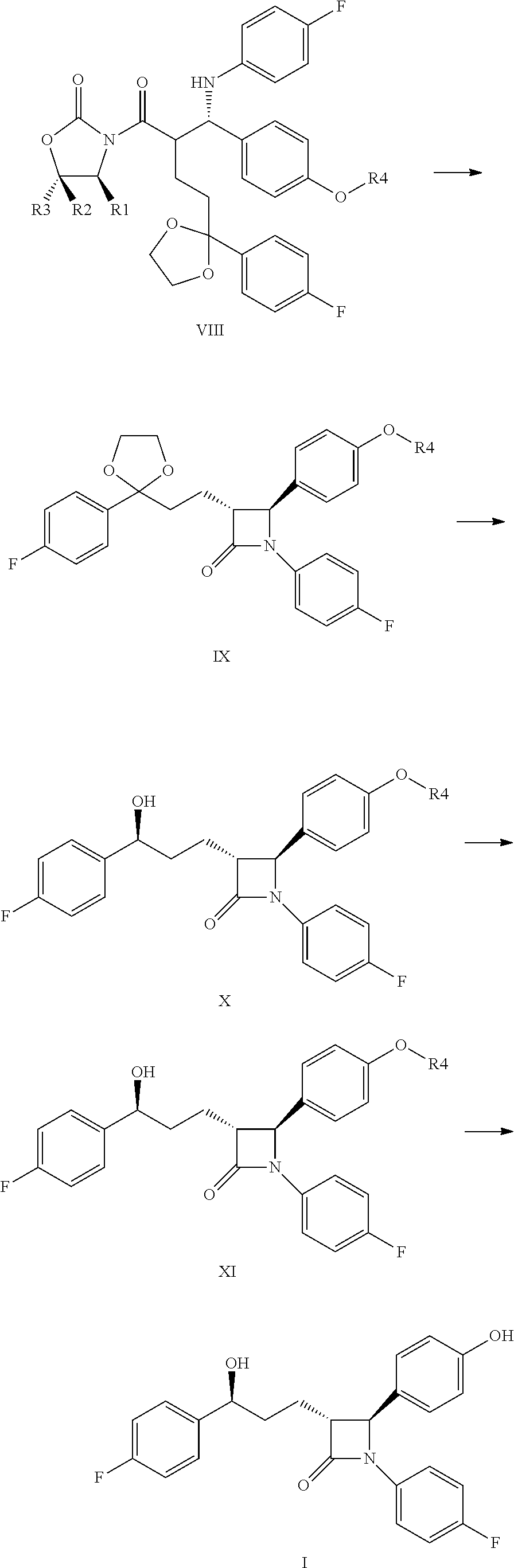
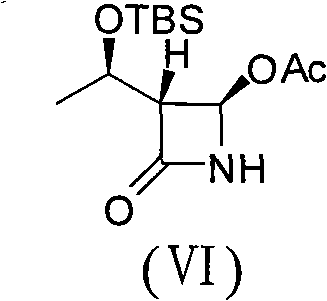
Process for the production of ezetimibe and intermediates used in this process
InactiveUS20090216009A1Efficient purificationImprove abilitiesSilicon organic compoundsGroup 3/13 element organic compoundsEzetimibePhotochemistry
An industrially easily realizable and economical process comprising only few steps, and built on new intermediates for the production of 1-(4-3(R)-[3(S)-(4-fluorophenyl)-3-hydroxypropyl]-4(S)-(4-hydroxyphenyl)-2-azetidinone (ezetimibe) according to the reaction scheme described herein.
Owner:RICHTER GEDEON NYRT
Synthetic method of (2S, 3S)-2-[(carbamoyloxy) methyl]-3-amido-4-oxoazetidinone-1-sulfonate
ActiveCN101298430AThe reaction steps are simpleHigh yieldAsymmetric synthesesMethyl carbamateSodium azide
The invention relates to a synthetic method of (2S, 3S)-2-[(carbamoyloxy) methyl]-3-amino-4-oxoazetidinone-1-sulfonate which includes the following steps: step one: dropping bromine into a solvent containing L-aspartic acid and obtaining (2R, 3R)-2-bromine-3-amino-succinic acid under the catalyzing of red phosphorus; step two: dropping thionyl chloride into the solvent containing naphthyridine for closed loop esterification; step three: using sodium azide to carry out heating reaction with (2R, 3R)-3-bromine-4-oxoazetidinone-2-methyl formate in the solvent; step four: using sodium borohydride to reduce (2R, 3S)-3-azide-4-oxoazetidinone-2-methyl formate in the solvent; step five: using chlorosulfonyl isocyanate to react with (3S, 4S)-3-azide-4- hydroxymethyl-2-azetidinone in the solvent; step six: using sulfur trioxide pyridine to completely react with (2S, 3S)-3-azide-4-oxoazetidinone-2-methyl carbamate in the solvent; step seven: obtaining the (2S, 3S)-2-[(carbamoyloxy) methyl]-3-amino-4- oxoazetidinone-1-sulfonate by Pd / C catalyzing and hydrogenation in the solvent.
Owner:上海立科化学科技有限公司
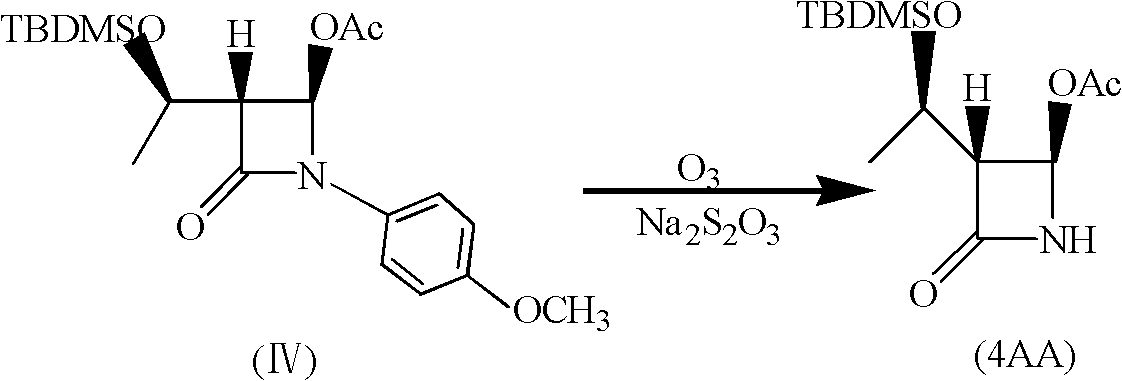
Method for reduction preparation of 4-AA with inorganic reducing agent
The invention discloses a method for reduction preparation of (3R,4R)-4-acetoxyl-3-[(R)-1-[(terbutyldimethylsilyl)oxy-ethyl]-2-azetidinone with an inorganic reducing agent. The inorganic reducing agent is reductive metal powder. The method includes the following steps that firstly, the inorganic reducing agent is pretreated, wherein the reductive metal powder is placed in an acidic or alkaline solution to be activated to obtain activated metal powder; secondly, ozone is introduced into a solution of a compound I for an oxidation reaction to obtain an ozonization reaction solution; thirdly, theactivated metal powder obtained in the first step is added into the ozonization reaction solution obtained in the second step for a reduction reaction, and (3R,4R)-4-acetoxyl-3-[(R)-1-[(terbutyldimethylsilyl)oxy-ethyl]-2-azetidinone is obtained, wherein the structural formula of the compound I is shown in the description. The method only needs one-step reduction, the amount of wastewater can be reduced, the content of ammonia nitrogen and sulfur in the wastewater is reduced, and the COD value of the wastewater is reduced.
Owner:JIANGSU HANKUO BIOLOGICAL
Process for stereoselective preparation of 4-bma using a chiral auxiliary
The present invention relates to a process for preparing (3R,4S)-3-[[[R]-V-t- butyldimethylsilyloxy] ethyl] -4- [(R)- l'-carboxyethyl]-2-azetidinone [4-BMA: formula (6)], a key intermediate for the synthesis of carbapenem and penem antibiotics. Specifically, the present invention relates to a process comprising first, the preparation of a chiral auxiliary from cheap L-Valinol, and then the preparation of 4-BMA in high yield and high selectivity, under industrially mild conditions.
Owner:DAEWOONG PHARM CO LTD +1
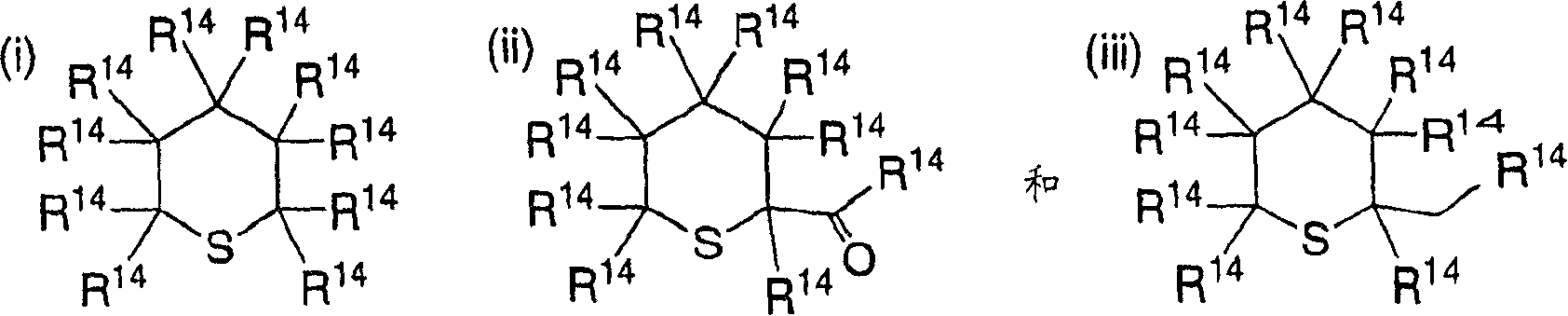
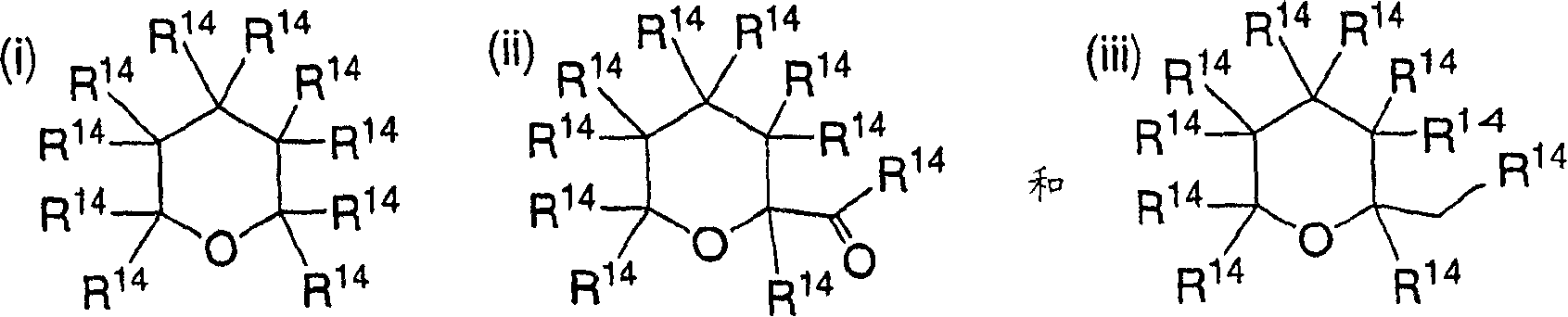
2-azetidinones as anti-hypercholesterolemic agents
InactiveCN1870988AAvoid absorptionOrganic active ingredientsSugar derivativesAtherothrombotic diseaseCholesterol absorption inhibitor
Owner:MERCK & CO INC
Process for the production of ezetimibe and intermediates used in this process
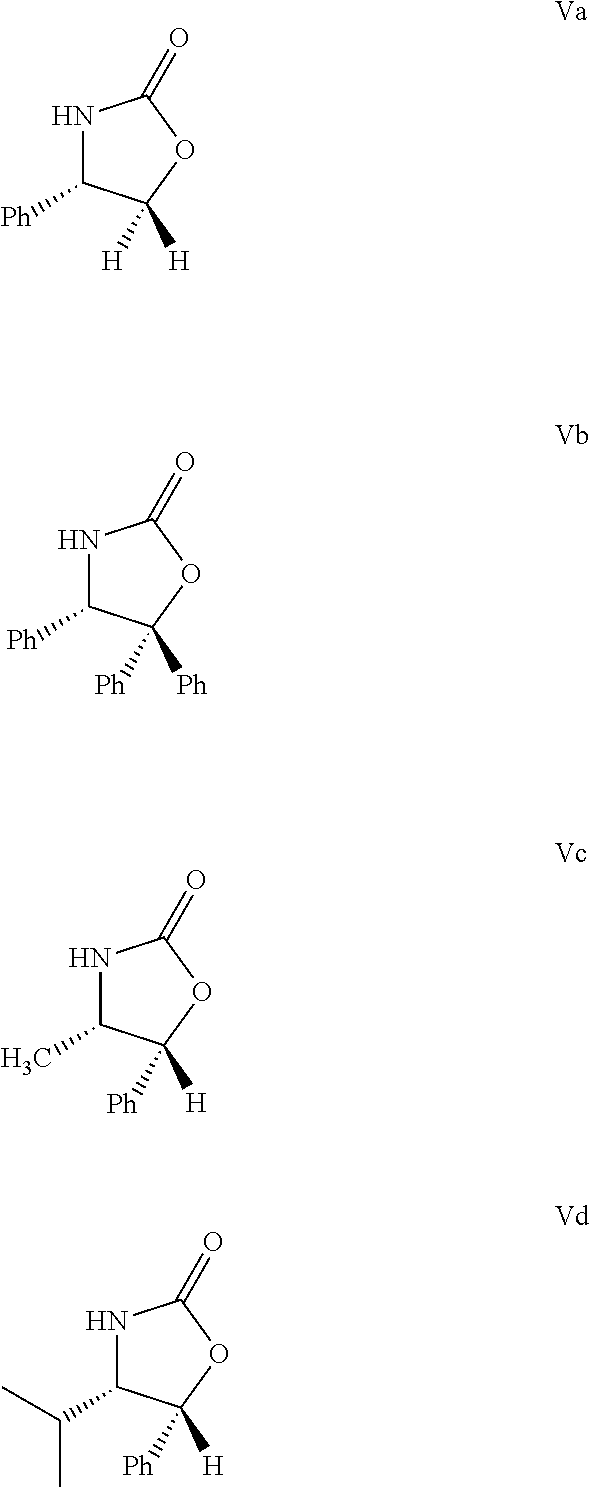
A process for the production of 1-(4-3(R)-[3(S)-(4-fluorophenyl)-3-hydroxypropyl]-4(S)-(4-hydroxyphenyl)-2-azetidinone (ezetimibe) according to the following reaction scheme: (I), (II), (III), (IV), (V), (VI), (VII), (VIII), (IX), (X), (XI) where the substances of the general Formulas II, IV, VI, VIII, IX, X and XI are new, Formula III is a non-isolated intermediate, R1, R2 and R3 are represented by the compounds of Formulas Va-Vd, (Va), (Vb), (Vc), (Vd) and R4 is a silyl, e.g., tert-butyl-dimethyl-silyl, tert-butyl-diphenyl-silyl group.
Owner:RICHTER GEDEON NYRT
(3S,4S)-4-acetyl-3-((R)-1-hydroxyethyl)-2-azetidinone and preparation method of same
InactiveCN101891665AEasy to purifyMild responseOrganic chemistryOrganic-compounds/hydrides/coordination-complexes catalystsEthyl phosphate2-Azetidinone
The invention provides (3S,4S)-4-acetyl-3-((R)-1-hydroxyethyl)-2-azetidinone and a preparation method of the same. The invention further provides the intermediates of (2R,3R)-N-benzhydryl-N-(2-oxopropyl)epoxy butyramide (I) and (3S,4S)-1-benzhydryl-3-((R)-1-hydroxyethyl)-4-acetyl-2-azetidinone (II) of the (3S,4S)-4-acetyl-3-((R)-1-hydroxyethyl)-2-azetidinone and preparation methods thereof. The method has the advantages of low-cost of easily obtained raw material, high yield, simple and convenient operation, environment friendliness and easy achievement of mass production.
Owner:SHANGHAI INST OF PHARMA IND
Synthesis of sulfomycin derivant
InactiveCN101367830AThe synthesis steps are simpleLower reaction costGroup 4/14 element organic compoundsTriethylphosphiteTert-Butyloxycarbonyl protecting group
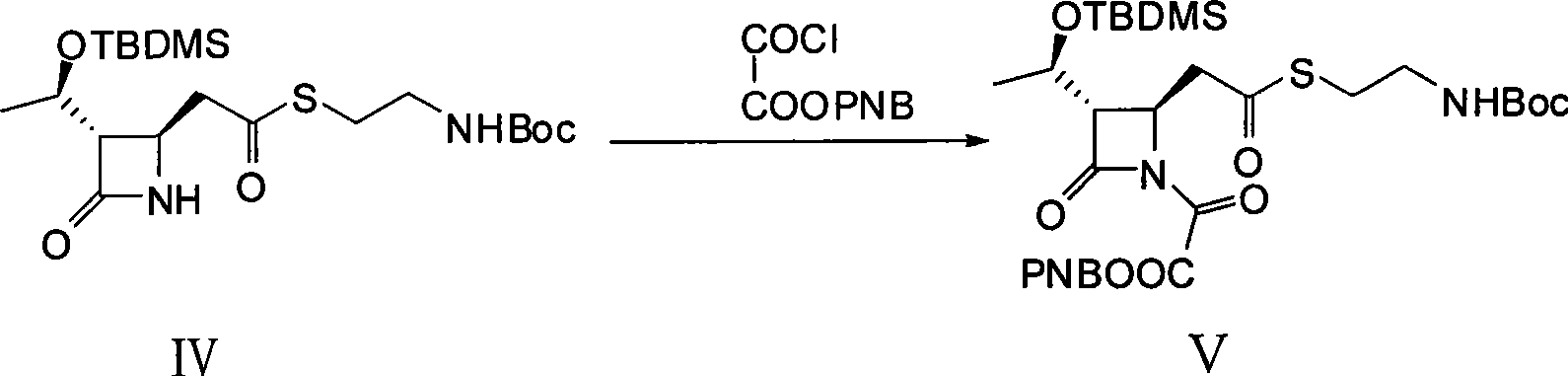
The present invention discloses a method for synthesizing a thienamycin derivative, which uses (3S, 4R)-3-[(1R)-1-(t-butyldilnethylsilyloxy)ethyl]-4-{2-[2-(Boc-amino)ethylsuleenyl]carboxyethyl}-2-azetidinone as a starting material and the commercialized triethyl phosphite as Wittig reagent and adopts two steps of reactions (acylation reaction and intramolecular Wittig reaction) to synthesize the thienamycin derivative; because the Wittig reagent does not need to be synthesized independently, the synthesization steps can be simplified, and the reaction cost can be reduced; and the starting material can be prepared from the commercialized (3S, 4R)-3-[(1R)-1-(t-butyldilnethylsilyloxy)ethyl]-4-acetoxy-2-azetidinone by three steps of reactions (allylation reaction, oxidation reaction and esterification reaction). The synthesis method has the advantages of short route, mild reaction conditions, ready availability of materials and low cost, and the obtained thienamycin derivative, which can be used as a midbody, is used for the synthesis of carbapenem antibiotics with pharmacological activity and the like.
Owner:CHONGQING UNIV
Synthesis method of (3R, 4R)-3-[(R)-1-tertiary butyl dimethyl Si-O-ethyl]-4-acetoxyl-2-azetidinone derivative
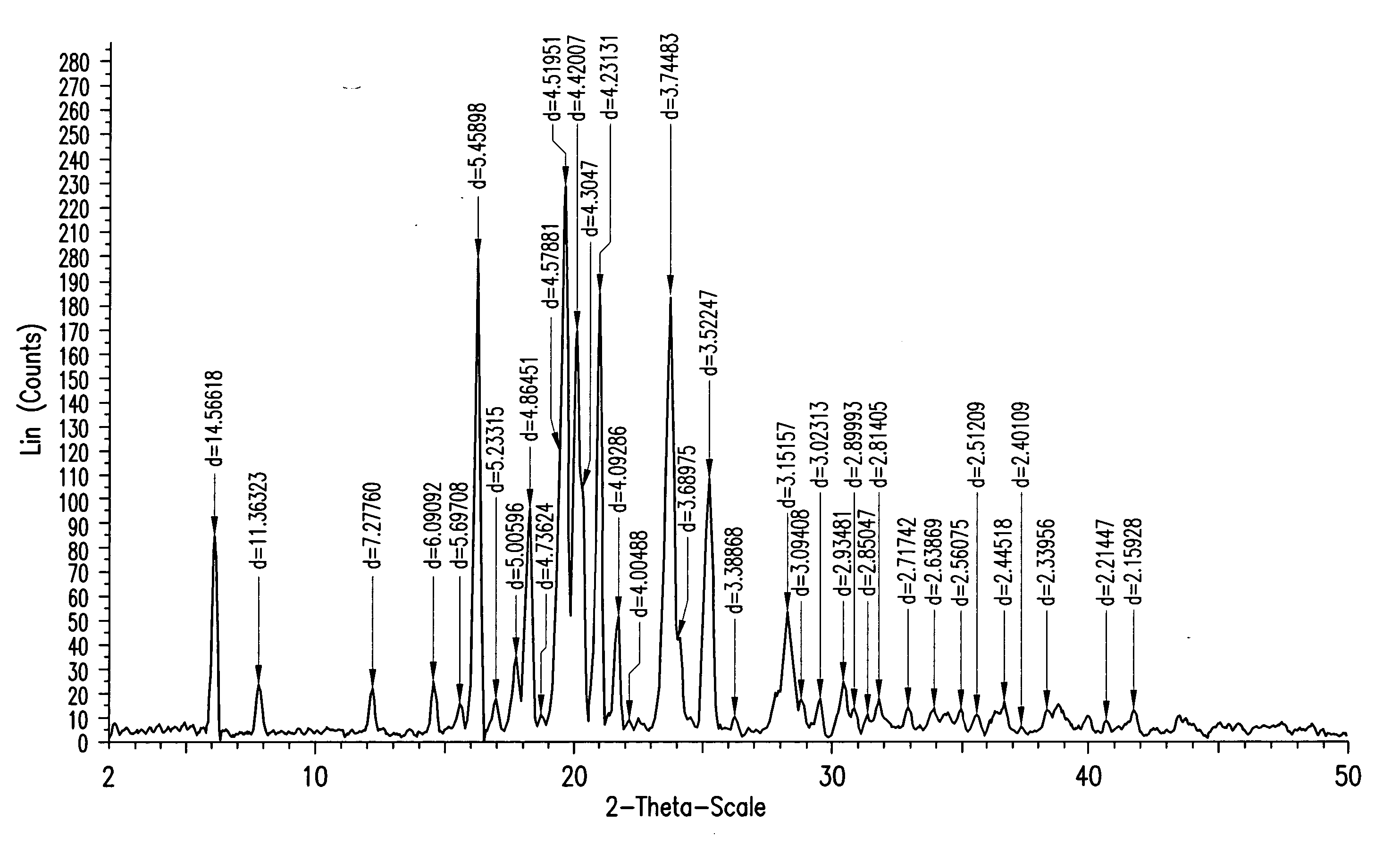
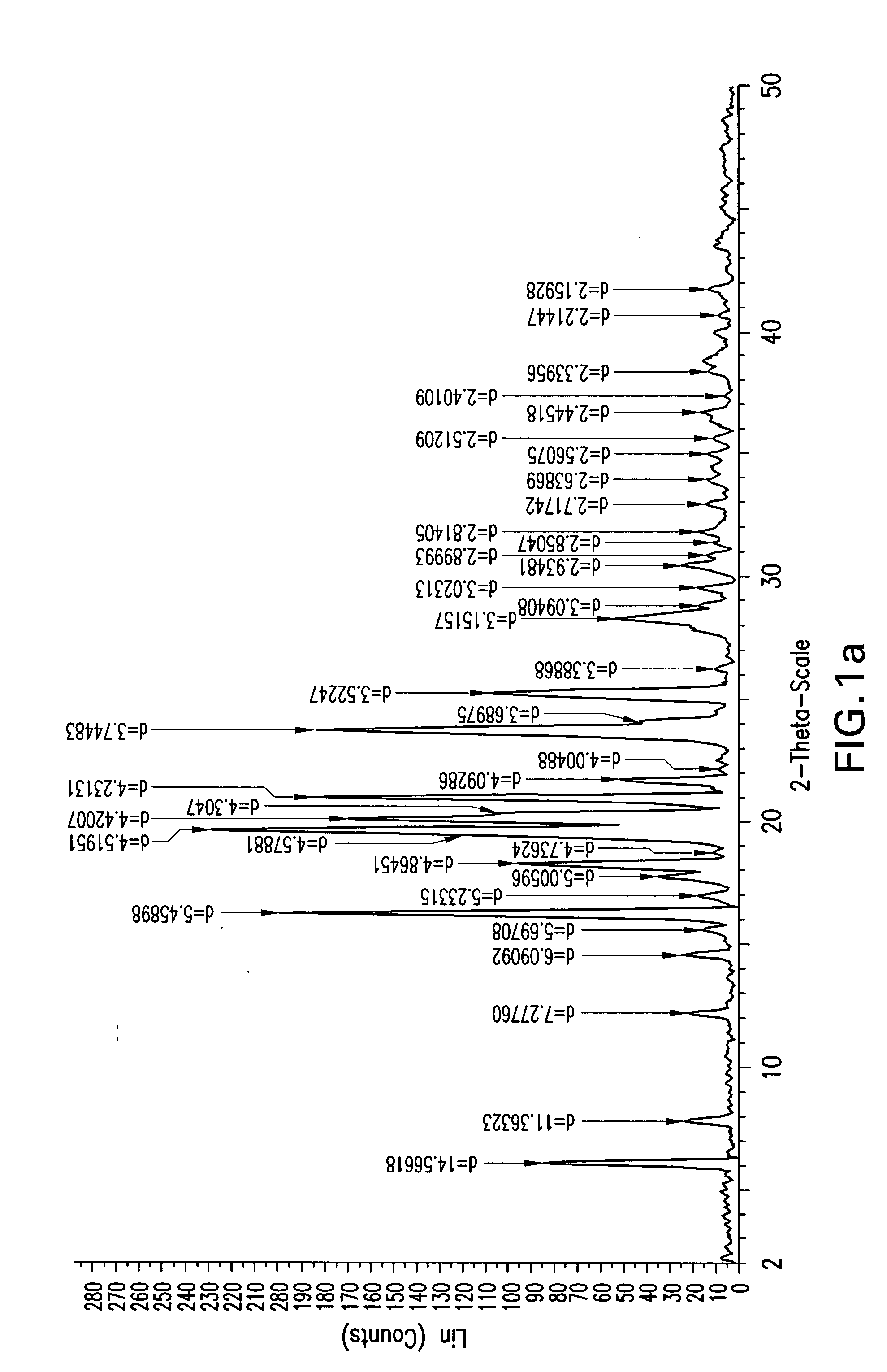
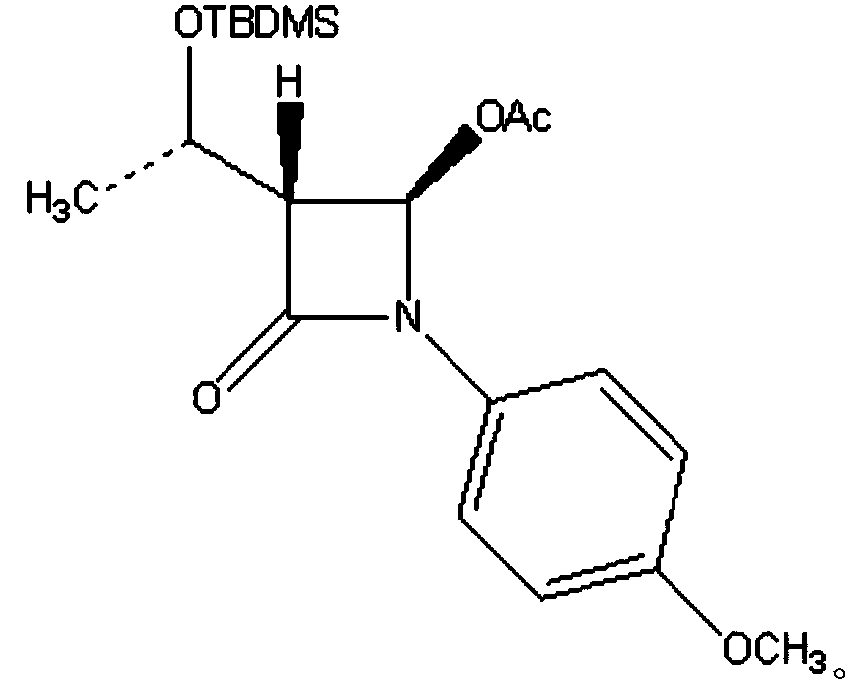
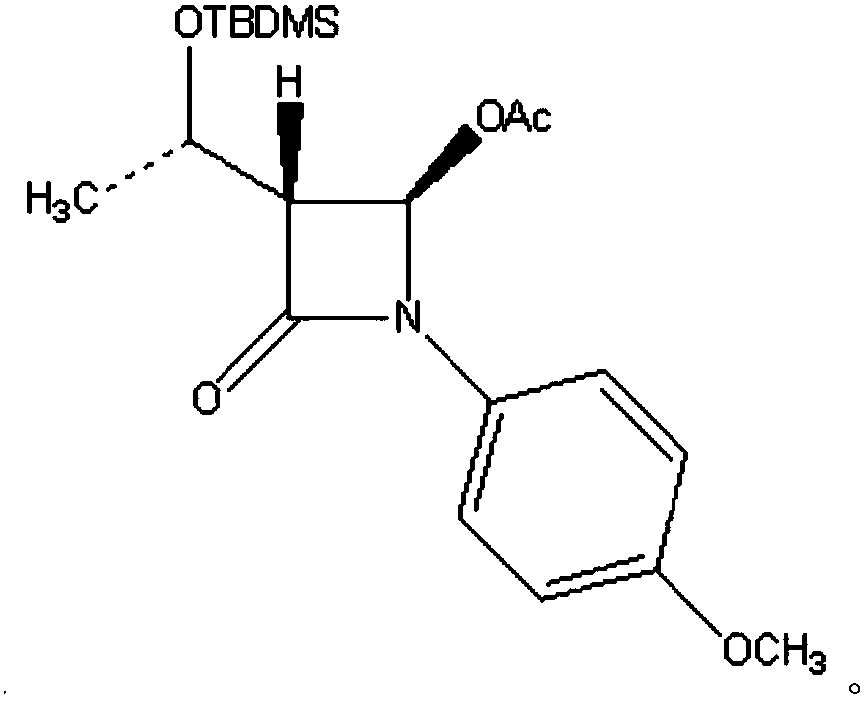
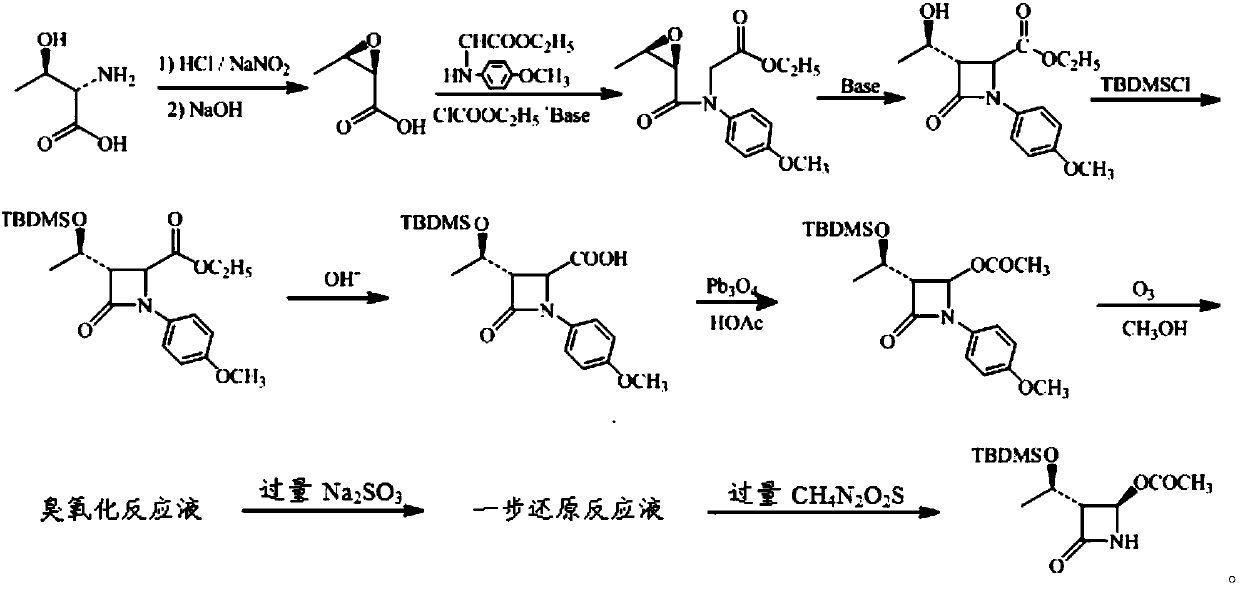
InactiveCN101863916ASubsequent synthesis safety without any impactEasy to operateGroup 4/14 element organic compoundsMetal/metal-oxides/metal-hydroxide catalystsSynthesis methodsHigh activity
The invention relates to a synthesis method of a (3R, 4R)-3-[(R)-1-tertiary butyl dimethyl Si-O-ethyl]-4-acetoxyl-2-azetidinone derivative. The derivative is prepared by carrying out a reflux reaction on 4-AA at 30-120 DEG C in an organic solvent by using zinc powder as a catalyst. Compared with the traditional beta-lactam antibiotic derivative using a larger group, the invention has the advantages that a smaller group easier to obtain is used for synthesizing the beta-lactam antibiotic derivative in the method, and the synthesis method has high activity, good selectivity and high atom economy. Raw materials used in the reaction have low toxicity and are easy to obtain, and a heavy metal catalyst is not needed to be used, which is safe and has no influence on subsequent pharmaceutical synthesis. The method has simple process, easy operation and moderate reaction condition.
Owner:JIAXING UNIV
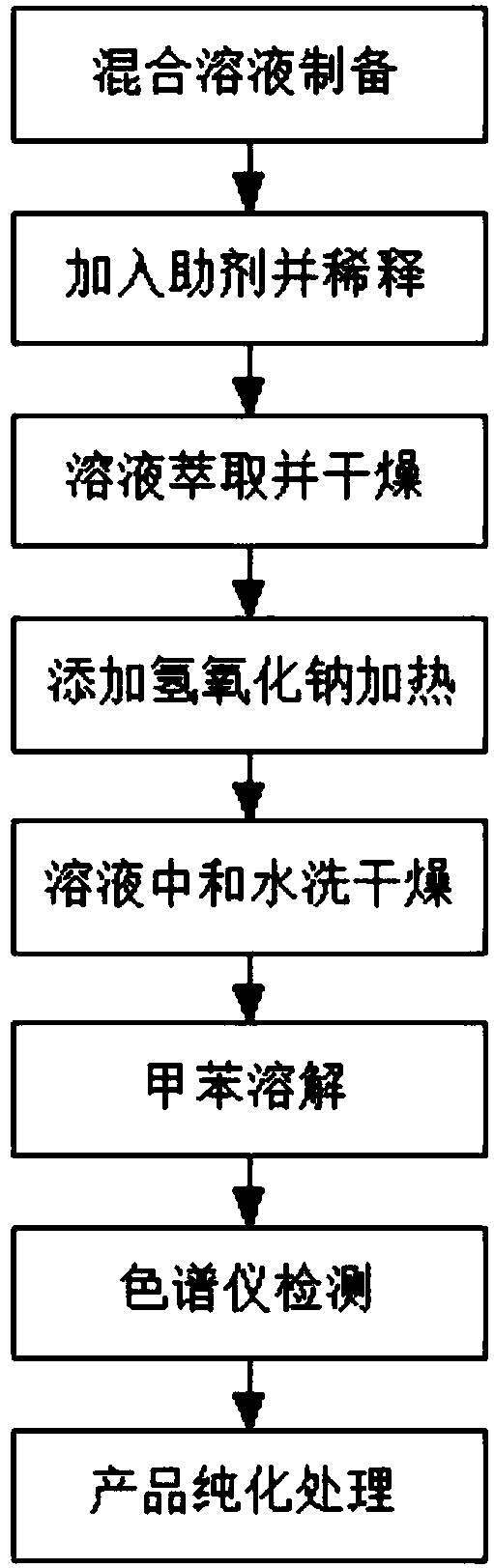
Method for synthesizing 2-azetidinone
The invention discloses a method for synthesizing 2-azetidinone in the technical field of chemical synthesis. The method comprises specific steps as follows: S1, preparation of a mixed solution; S2, addition of an aid and dilution; S3, solution extraction and drying; S4, addition of sodium hydroxide and heating; S5, solution neutralization, water washing and drying; S6, toluene dissolution; S7, detection with a chromatograph; S8, product purification treatment. The synthesis method is simple, the prepared finished product has high accuracy, the temperature and the reaction time are controllable in the synthesis process, no raw material waste phenomenon is caused in the synthesis process and the method is suitable for large-scale production in a factory.
Owner:ZHONGSHAN DEGAOXING INTPROP CENT LLP
Synthesis method of 4-acetoxyl-2-azetidinone
ActiveCN102002066BReduce use costReduce pollutionGroup 4/14 element organic compoundsBulk chemical productionSynthesis methodsEconomic benefits
A synthesis method of 4-acetoxyl-2-azetidinone comprises the following steps of: (1) taking N-p-methoxypheny-N-(acetyl) methyleneimine as a raw material and carrying out cyclization reaction with (R)3-tert-butyldimethylsilyloxy-sulfo-butyrate-S-2-pyridinyl ester to obtain (3S,4S)-3-[(1'R)-tert-butyldimethylsilyloxyethyl]-4-acetyl-1-p-methoxyphenyl-2-azetidinone; (2) preparing (3R,4R)-3-[(1'R)-tert-butyldimethylsilyloxyethyl]-4-acetoxyl -1-p-methoxyphenyl-2-azetidinone from peroxyacetic acid through oxidization in the presence of Na3PO4 and a phase transfer catalyst; and (3) removing protective groups to obtain a final product (3R,4R)-3-[(1'R)-tert-butyldimethylsilyloxyethyl]-4-acetoxyl-2-azetidinone. The invention has the advantages of shortening reaction period, improving reaction total yield, and improving reaction yield and product purity by introducing the phase transfer catalyst in the step (2). The method is simple, causes small pollution and has great application value and economic benefit.
Owner:YIYUAN XINQUAN CHEM
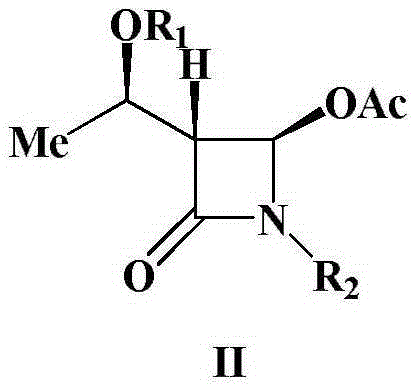
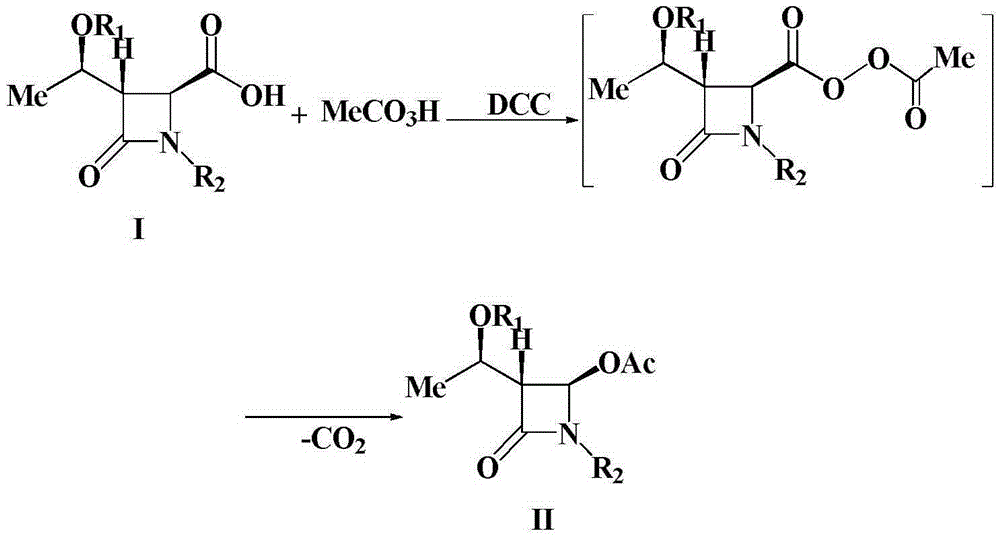
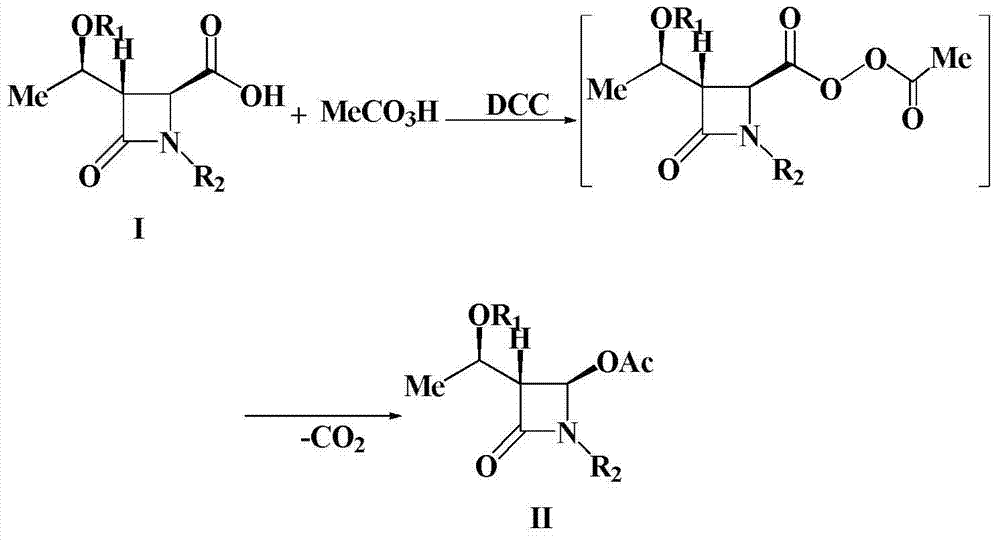
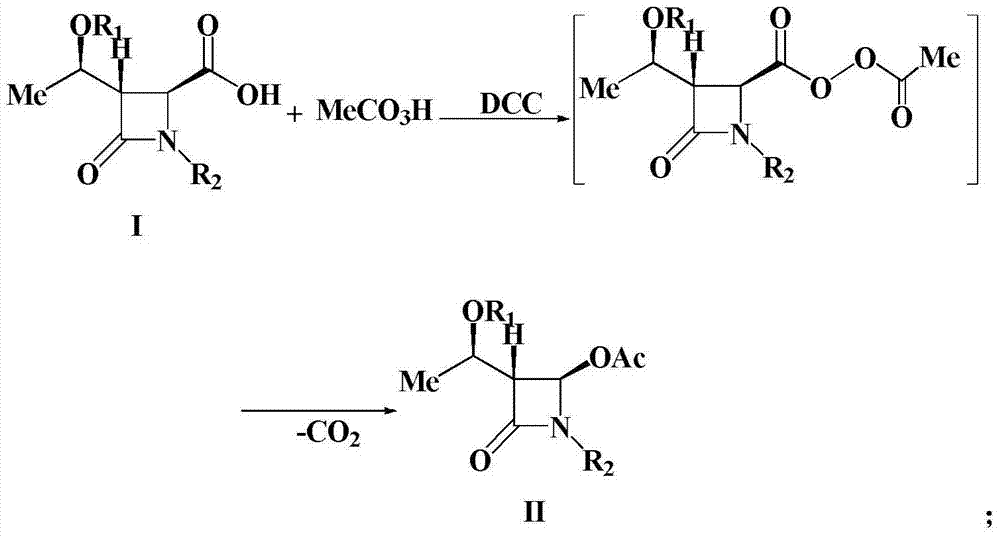
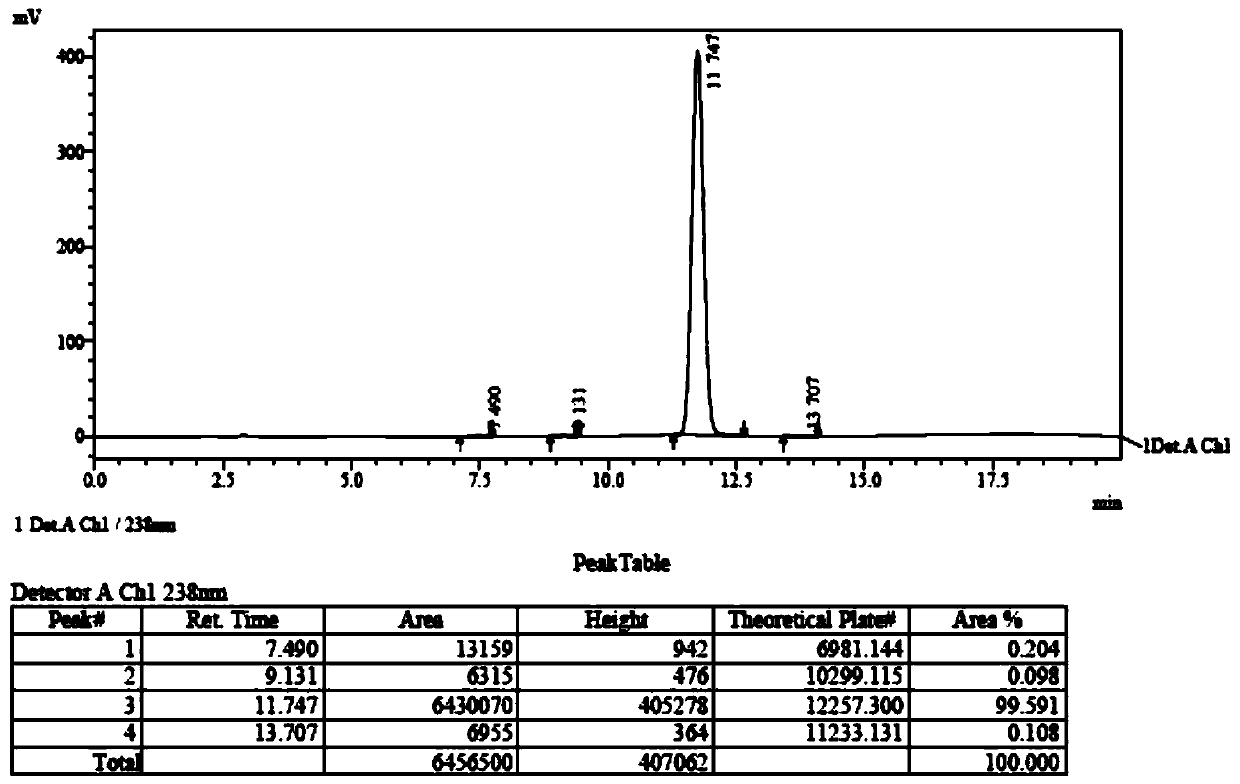
A kind of preparation method of 4-acetoxy-2-azetidinone compound
InactiveCN103539813BResidue reductionEmission reductionOrganic chemistry4-carboxy-2-azetidinone2-butanone
The invention relates to a preparation method of 4-acetyloxy-2-azetidinone compounds, and is used for solving the problem that the large-scale industrial production cannot be favorably realized because of defects such as severe reaction condition, complex treatment process, high cost, serious environment pollution and the like in the prior art. The method provided by the invention comprises the step of carrying out oxidization deacidification in the existence of a dehydrating agent N, N'-dicyclohexylcarbodiimide and an oxidizing agent peroxyacetic acid by taking 4-carboxyl-2-azetidinone compounds I as raw materials to obtain the 4-acetyloxy-2-azetidinone compounds II. A heavy metal oxidizing agent or catalyst is prevented from being used in the oxidization deacidification process, so that the residue and discharge of heavy metals and environmental pollution caused by the heavy metals are greatly reduced. Byproducts generated in a reaction process can be conveniently recycled, so that the production cost is reduced to the great extent, and the preparation method is more excellent on the aspect of economical efficiency. The method is mild in reaction condition, simple and convenient to operate and suitable for large-scale production; the 4-acetyloxy-2-azetidinone compounds are easy to separate and purify and high in yield.
Owner:TAIZHOU VOCATIONAL & TECHN COLLEGE +1
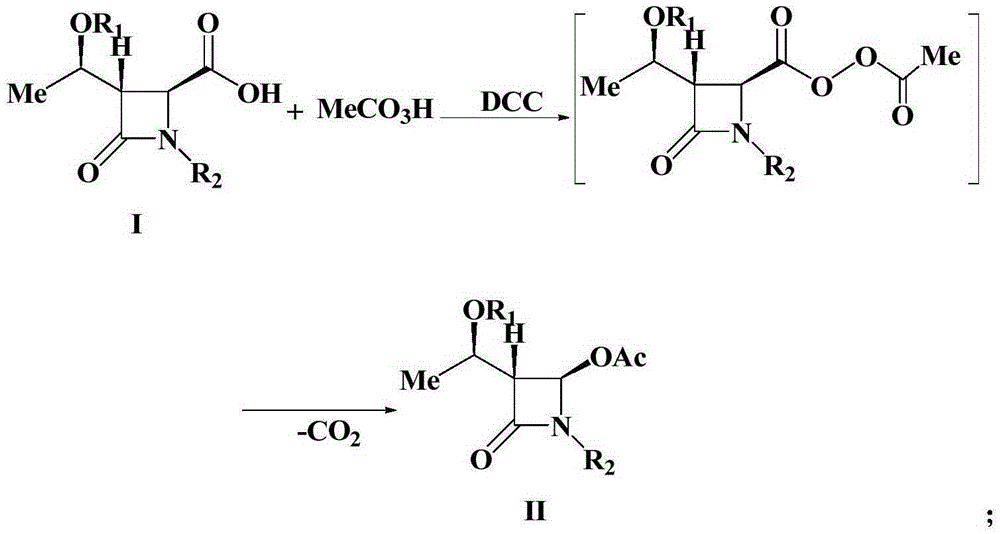
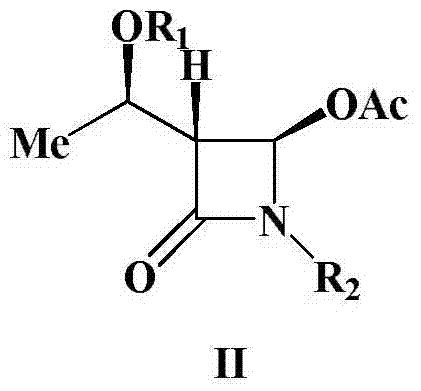
Preparation method of 4-acetyloxy-2-azetidinone compounds
InactiveCN103539813AResidue reductionEmission reductionGroup 4/14 element organic compounds4-carboxy-2-azetidinoneOxidizing agent
The invention relates to a preparation method of 4-acetyloxy-2-azetidinone compounds, and is used for solving the problem that the large-scale industrial production cannot be favorably realized because of defects such as severe reaction condition, complex treatment process, high cost, serious environment pollution and the like in the prior art. The method provided by the invention comprises the step of carrying out oxidization deacidification in the existence of a dehydrating agent N, N'-dicyclohexylcarbodiimide and an oxidizing agent peroxyacetic acid by taking 4-carboxyl-2-azetidinone compounds I as raw materials to obtain the 4-acetyloxy-2-azetidinone compounds II. A heavy metal oxidizing agent or catalyst is prevented from being used in the oxidization deacidification process, so that the residue and discharge of heavy metals and environmental pollution caused by the heavy metals are greatly reduced. Byproducts generated in a reaction process can be conveniently recycled, so that the production cost is reduced to the great extent, and the preparation method is more excellent on the aspect of economical efficiency. The method is mild in reaction condition, simple and convenient to operate and suitable for large-scale production; the 4-acetyloxy-2-azetidinone compounds are easy to separate and purify and high in yield.
Owner:TAIZHOU VOCATIONAL & TECHN COLLEGE +1
4AA intermediate refining method
The invention discloses a 4AA intermediate refining method. According to the refining method, a 4AA intermediate (III) to be refined is added into a mixed solvent system composed of a solvent A and asolvent , after the 4AA intermediate is dissolved, the solution is extracted, layering is performed, the phase containing the 4AA intermediate (III) is crystallized to obtain the refined 4AA intermediate (III). The provided refining method can remove organic impurities in the 4AA intermediate (III) namely (3S,4S)-1-(4-methoxylphenyl)-3-[((R)-1-hydroxylethyl)]-4-acetyl-2-azetidinone; the refining yield is high, the purity is high, the influence of impurities on subsequent reactions is eliminated, the using amount of organic reagents in subsequent reactions is reduced, and the 4AA product quality is improved.
Owner:JIANGXI FUSHINE PHARMA CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
![Method for preparing (3R,4R)-3-[(1R)tert-butyl-dimethyl-silyloxyethyl]-4-acetoxyl-2-azetidinone Method for preparing (3R,4R)-3-[(1R)tert-butyl-dimethyl-silyloxyethyl]-4-acetoxyl-2-azetidinone](https://images-eureka.patsnap.com/patent_img/14cc60c7-7167-4ded-b95b-fbb8a744c5ad/152649DEST_PATH_IMAGE004.PNG)
![Method for preparing (3R,4R)-3-[(1R)tert-butyl-dimethyl-silyloxyethyl]-4-acetoxyl-2-azetidinone Method for preparing (3R,4R)-3-[(1R)tert-butyl-dimethyl-silyloxyethyl]-4-acetoxyl-2-azetidinone](https://images-eureka.patsnap.com/patent_img/14cc60c7-7167-4ded-b95b-fbb8a744c5ad/184190DEST_PATH_IMAGE001.PNG)
![Method for preparing (3R,4R)-3-[(1R)tert-butyl-dimethyl-silyloxyethyl]-4-acetoxyl-2-azetidinone Method for preparing (3R,4R)-3-[(1R)tert-butyl-dimethyl-silyloxyethyl]-4-acetoxyl-2-azetidinone](https://images-eureka.patsnap.com/patent_img/14cc60c7-7167-4ded-b95b-fbb8a744c5ad/359203DEST_PATH_IMAGE005.PNG)
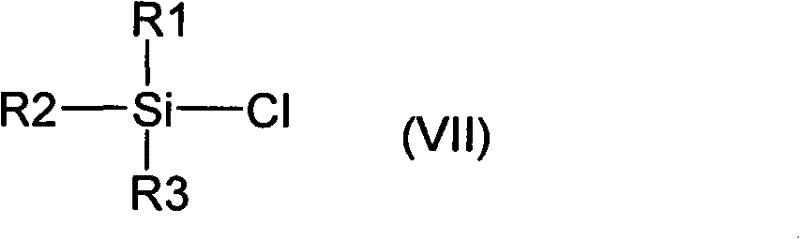
![Intermediates for the preparation of (3r, 4s)-1-(4-fluorophenyl)-3-[(3S)-3-(4-fluorophenyl)-3-hydroxypropyl)]-4-(4-hydroxyphenyl)-2-azetidinone Intermediates for the preparation of (3r, 4s)-1-(4-fluorophenyl)-3-[(3S)-3-(4-fluorophenyl)-3-hydroxypropyl)]-4-(4-hydroxyphenyl)-2-azetidinone](https://images-eureka.patsnap.com/patent_img/4329719b-1b73-4e18-9ef9-6e95a9db0c99/US20110046389A1-20110224-C00001.png)
![Intermediates for the preparation of (3r, 4s)-1-(4-fluorophenyl)-3-[(3S)-3-(4-fluorophenyl)-3-hydroxypropyl)]-4-(4-hydroxyphenyl)-2-azetidinone Intermediates for the preparation of (3r, 4s)-1-(4-fluorophenyl)-3-[(3S)-3-(4-fluorophenyl)-3-hydroxypropyl)]-4-(4-hydroxyphenyl)-2-azetidinone](https://images-eureka.patsnap.com/patent_img/4329719b-1b73-4e18-9ef9-6e95a9db0c99/US20110046389A1-20110224-C00002.png)
![Intermediates for the preparation of (3r, 4s)-1-(4-fluorophenyl)-3-[(3S)-3-(4-fluorophenyl)-3-hydroxypropyl)]-4-(4-hydroxyphenyl)-2-azetidinone Intermediates for the preparation of (3r, 4s)-1-(4-fluorophenyl)-3-[(3S)-3-(4-fluorophenyl)-3-hydroxypropyl)]-4-(4-hydroxyphenyl)-2-azetidinone](https://images-eureka.patsnap.com/patent_img/4329719b-1b73-4e18-9ef9-6e95a9db0c99/US20110046389A1-20110224-C00003.png)
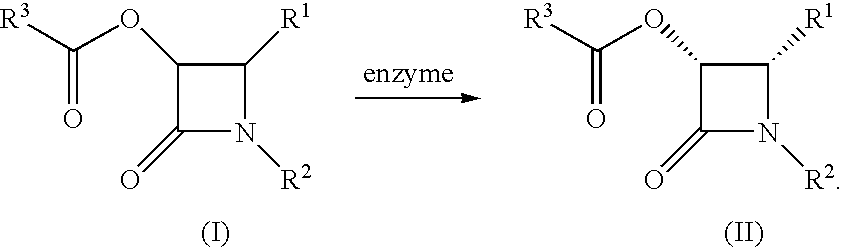

![Synthetic method of (2S, 3S)-2-[(carbamoyloxy) methyl]-3-amido-4-oxoazetidinone-1-sulfonate Synthetic method of (2S, 3S)-2-[(carbamoyloxy) methyl]-3-amido-4-oxoazetidinone-1-sulfonate](https://images-eureka.patsnap.com/patent_img/59b875e2-6f92-4cd0-87d3-6a279cc60621/a20081003896200051.PNG)
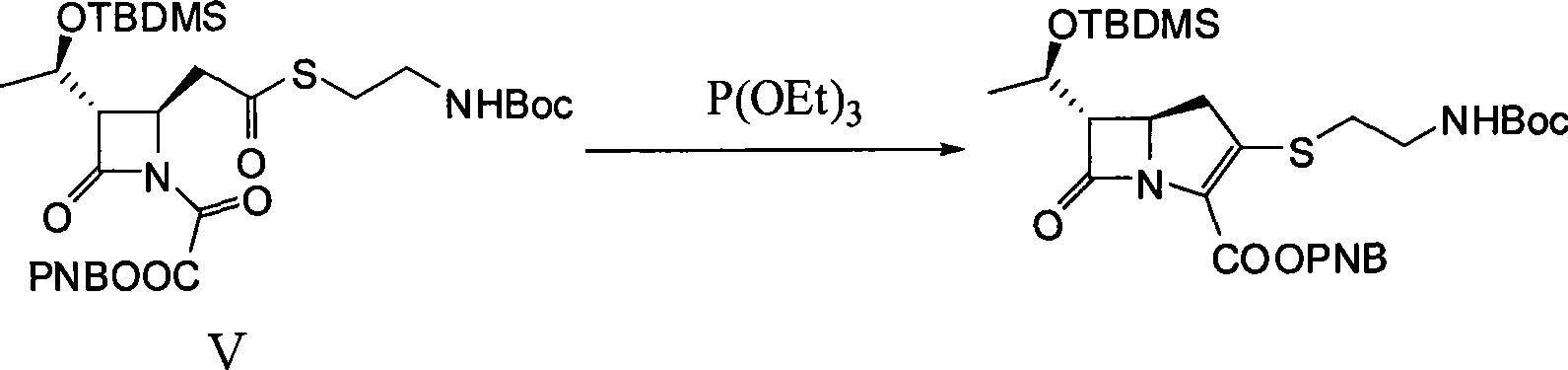
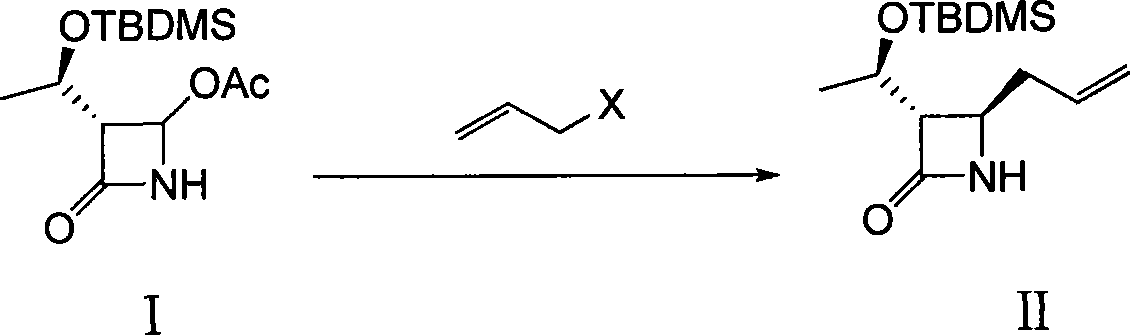
![Synthesis method of (3R, 4R)-3-[(R)-1-tertiary butyl dimethyl Si-O-ethyl]-4-acetoxyl-2-azetidinone derivative Synthesis method of (3R, 4R)-3-[(R)-1-tertiary butyl dimethyl Si-O-ethyl]-4-acetoxyl-2-azetidinone derivative](https://images-eureka.patsnap.com/patent_img/07094fb8-a05f-47b3-ab0c-ec7a0293758e/000001.png)
![Synthesis method of (3R, 4R)-3-[(R)-1-tertiary butyl dimethyl Si-O-ethyl]-4-acetoxyl-2-azetidinone derivative Synthesis method of (3R, 4R)-3-[(R)-1-tertiary butyl dimethyl Si-O-ethyl]-4-acetoxyl-2-azetidinone derivative](https://images-eureka.patsnap.com/patent_img/07094fb8-a05f-47b3-ab0c-ec7a0293758e/000002.png)
![Synthesis method of (3R, 4R)-3-[(R)-1-tertiary butyl dimethyl Si-O-ethyl]-4-acetoxyl-2-azetidinone derivative Synthesis method of (3R, 4R)-3-[(R)-1-tertiary butyl dimethyl Si-O-ethyl]-4-acetoxyl-2-azetidinone derivative](https://images-eureka.patsnap.com/patent_img/07094fb8-a05f-47b3-ab0c-ec7a0293758e/000003.png)