Method for synthesizing 2-azetidinone
A technology of azetidinone and solution, which is applied in the field of synthesizing 2-azetidinone, can solve the problems of difficult control of precision, low purity of 2-azetidinone, numerous uncontrollable factors, etc. The effect of high precision of finished product, controllable temperature and reaction time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
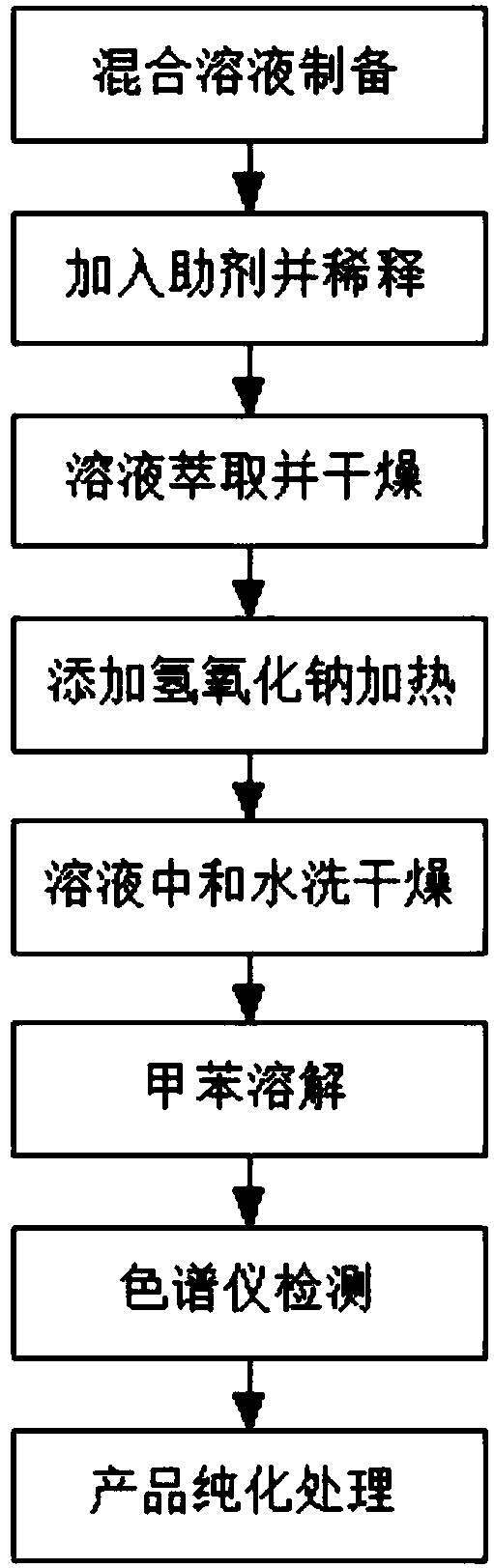
[0020] A method for synthesizing 2-azetidinone, the specific steps of the method are as follows:
[0021] S1: Add 10g of D-methyl lactate and 15g of bromochloromethane into the reaction flask, add 20ml of 4-dimethylaminopyridine solution, stir for 30min, and mix them uniformly to form a mixed solution;
[0022] S2: Cool the mixed solution to 0°, then slowly add the triethyl phosphite solution dropwise, after the addition is completed, remove the cooling device, raise the temperature to room temperature and react for 4 hours, after the reaction is complete, dilute the reaction solution with 100ml of pure water;
[0023] S3: The reaction solution was extracted with 60 ml of ether solution, dried over anhydrous sodium sulfate, and the solvent was removed to obtain an intermediate product;
[0024] S4: Add 5g of sodium hydroxide solution to the intermediate product, and heat the reaction, the temperature of the heating reaction is 50°C, and the reaction time is 1h;
[0025] S5: A...
Embodiment 2
[0030] A method for synthesizing 2-azetidinone, the specific steps of the method are as follows:
[0031] S1: Add 15g of D-methyl lactate and 20g of bromochloromethane into the reaction flask, add 25ml of 4-dimethylaminopyridine solution, stir for 30min, and mix them uniformly to form a mixed solution;
[0032] S2: Cool the mixed solution to 3°, then slowly add the triethyl phosphite solution dropwise, after the addition is completed, remove the cooling device, raise the temperature to room temperature and react for 5 hours, after the reaction is complete, dilute the reaction solution with 200ml of pure water;
[0033] S3: The reaction solution was extracted with 80 ml of diethyl ether solution, dried over anhydrous sodium sulfate, and the solvent was removed to obtain an intermediate product;
[0034] S4: Add 10 g of sodium hydroxide solution to the intermediate product, and heat the reaction. The temperature of the heating reaction is 60° C., and the reaction time is 1.5 h; ...
Embodiment 3
[0040] A method for synthesizing 2-azetidinone, the specific steps of the method are as follows:
[0041] S1: Add 13g of methyl D-lactate and 18g of bromochloromethane into the reaction flask, add 23ml of 4-dimethylaminopyridine solution, stir for 40min, and mix them uniformly to form a mixed solution;
[0042] S2: Cool the mixed solution to 2°, then slowly add the triethyl phosphite solution dropwise, after the addition is completed, remove the cooling device, raise the temperature to room temperature and react for 5 hours, after the reaction is complete, dilute the reaction solution with 150ml of pure water;
[0043] S3: The reaction solution was extracted with 70 ml of ether solution, dried over anhydrous sodium sulfate, and the solvent was removed to obtain an intermediate product;
[0044] S4: Add 7g of sodium hydroxide solution to the intermediate product, and heat the reaction, the temperature of the heating reaction is 55°C, and the reaction time is 1.5h;
[0045] S5:...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com