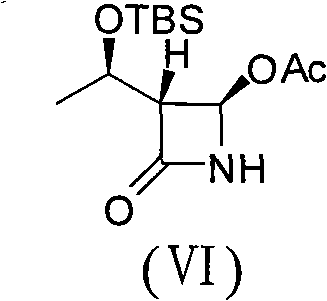
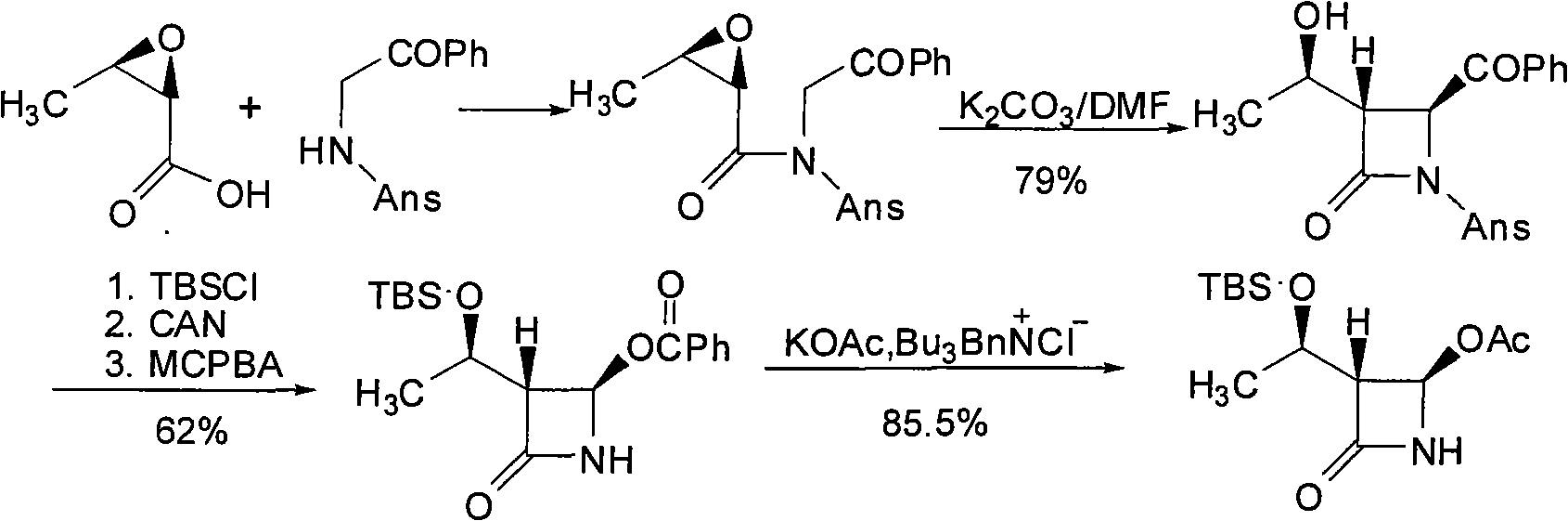
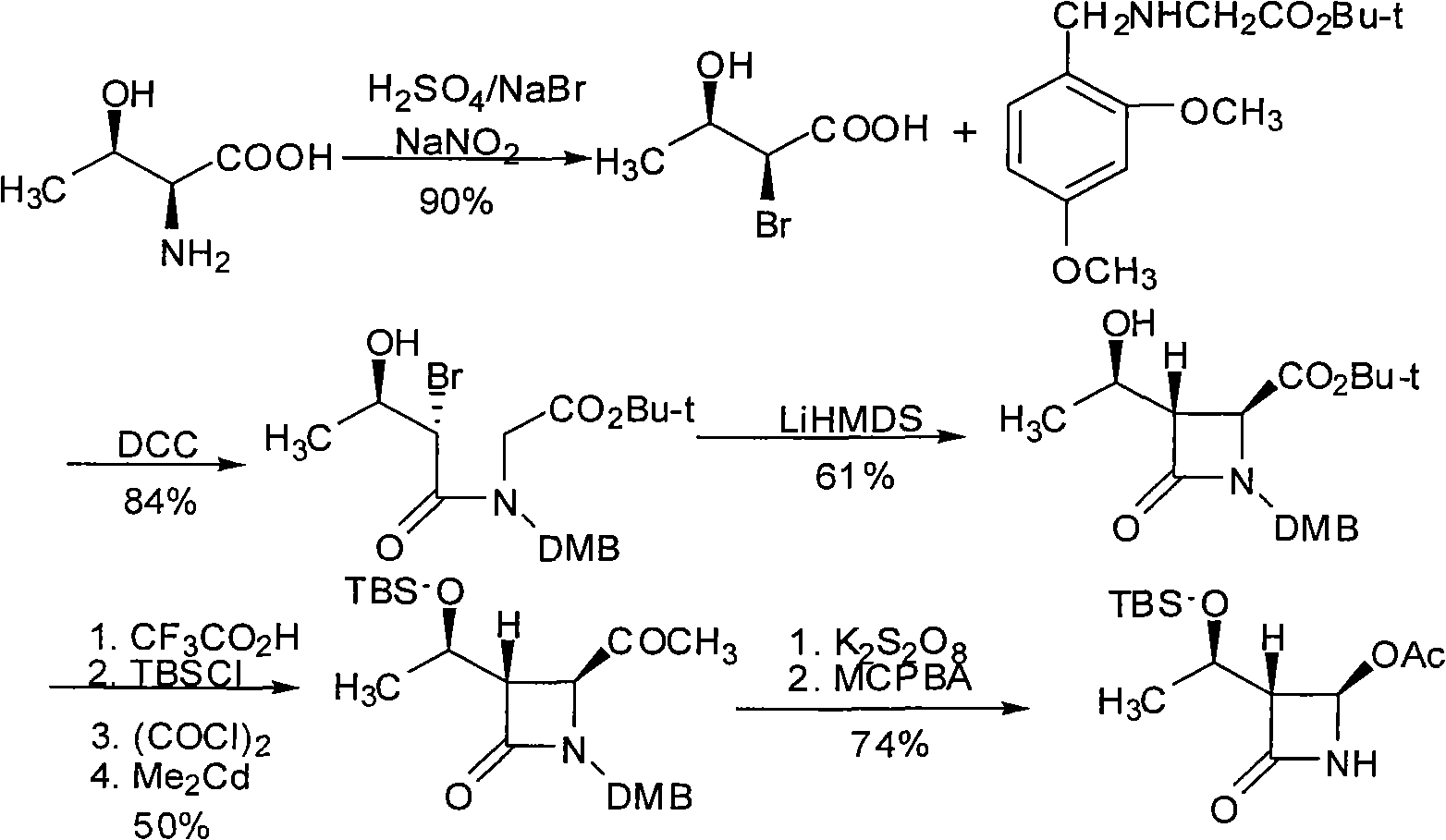
(3S,4S)-4-acetyl-3-((R)-1-hydroxyethyl)-2-azetidinone and preparation method of same
A technology of azetidinone and hydroxyethyl, which is applied in the field of medicinal chemistry, can solve problems such as difficult separation, difficult synthesis, and difficult availability in the market, and achieve the effects of simple post-treatment, mild reaction, and concise route
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Embodiment 1: the preparation of (2R, 3R)-N-benzhydryl-N-(2-oxopropyl) epoxybutanamide (formula (I) compound)
[0042] In a 250 mL four-neck round bottom flask equipped with a magnetic stirrer and a thermometer, add 7.9 g of sodium (2R,3R)-2,3-epoxybutyrate and 100 mL of tetrahydrofuran. After cooling to -15°C, add 3.7 mL of oxalyl chloride dropwise, stir at this temperature for 2 hours, add 5.3 mL of anhydrous pyridine, 6.4 g of 1-benzhydrylamino-2-propanone, stir for 1 hour and then rise to Stirring was continued at room temperature for 2 hours, 100 mL of ethyl acetate was added, washed with 5% sodium bicarbonate solution, the organic phase was washed with brine after liquid separation, dried over anhydrous magnesium sulfate, filtered, and the solvent was evaporated, and the compound (I) was obtained by silica gel column chromatography 7.2 g, yield 85.6%.
[0043]MS: 324.2 (M+H);
[0044] 1 HNMR: (400MHz, CDCl 3 )1.18(3H, m), 2.13(3H, s), 2.93(1H, m), 3.47(1H, m),...
Embodiment 2
[0045] Embodiment 2: the preparation of (2R, 3R)-N-benzhydryl-N-(2-oxopropyl) epoxybutyramide (formula (I) compound)
[0046] In a 250 mL four-necked round bottom flask equipped with a magnetic stirrer and a thermometer, 7.9 g of sodium (2R,3R)-2,3-epoxybutyrate and 100 mL of dichloromethane were added. After cooling to -20°C, add 4.9 g of thionyl chloride dropwise, stir at this temperature for 2 hours, add 10.5 g of triethylamine, 6.4 g of 1-benzhydrylamino-2-propanone, and stir for 1 hour Heat up to reflux reaction for 2 hours, add 100mL ethyl acetate, wash with 5% sodium bicarbonate solution, wash the organic phase with brine after liquid separation, dry over anhydrous magnesium sulfate, evaporate the solvent after filtration, and obtain compound (I )7.2g, yield 82.0%.
[0047] MS: 324.2 (M+H);
[0048] 1 HNMR: (400MHz, CDCl 3 )1.18(3H, m), 2.13(3H, s), 2.93(1H, m), 3.47(1H, m), 4.38(1H, s), 6.16(1H, s), 7.10-7.33(10H, m ).
Embodiment 3
[0049] Example 3: (3S, 4S)-1-benzhydryl-3-((R)-1-hydroxyethyl)-4-acetyl-2-azetidinone (compound of formula (II)) preparation of
[0050] In a 500 mL four-necked round bottom flask equipped with a magnetic stirrer and a thermometer, 7.0 g of the compound of formula (I) and 250 mL of dichloromethane were added. After cooling to -20°C, add LDA (1mol / L) 26.4mL dropwise, heat up to reflux for 3 hours after the dropwise addition, add 1N HCl 30mL, ethyl acetate 150mL, and use 5% sodium bicarbonate solution for the organic phase after liquid separation Washing, washing with brine, drying over anhydrous magnesium sulfate, filtering and distilling off the solvent, silica gel column chromatography to obtain compound (II) 5.4g, yield 77.4%.
[0051] MS: 324.2(M+H), 346(M+Na)
[0052] 1 HNMR: (400MHz, CDCl 3 )1.28(3H, m), 1.80(3H, s), 2.96(1H, m), 4.24(1H, m), 4.56(1H, s), 5.90(1H, s), 7.25-7.33(10H, m )
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com