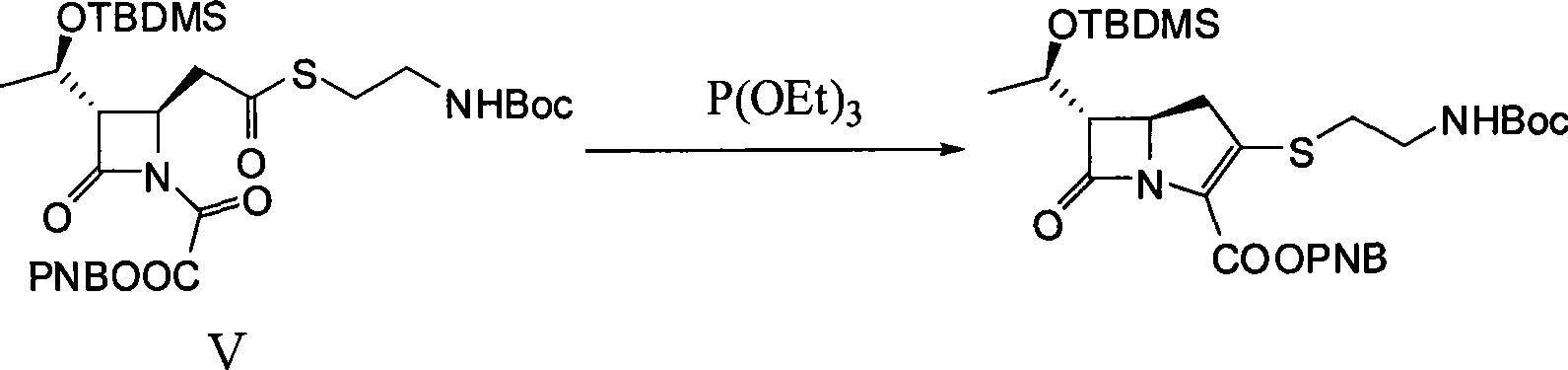Synthesis of sulfomycin derivant
A synthesis method and a derivative technology are applied in the synthesis field of thiomycin derivatives, which can solve the problems of unseen industrialized production, harsh reaction conditions, long synthesis routes and the like, and achieve the advantages of low cost, easy availability of raw materials and reduced reaction cost. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Embodiment one, the synthesis of thiamycin derivative
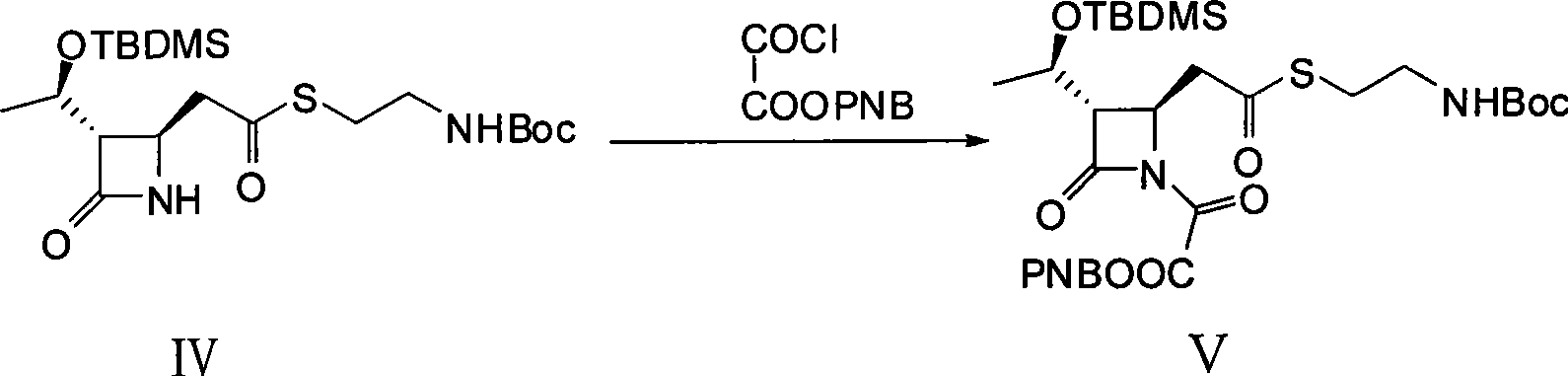
[0029] 1. (3S, 4R)-3-[(1R)-1-(tert-butyldimethylsilyloxy)ethyl]-4-{2-[2-(tert-butoxycarbonylamino)ethylthio Synthesis of ]carboxyethyl}-2-azetidinone-1-oxoacetic acid p-nitrobenzyl ester (V)
[0030] Dissolve 9 g (100 mmol) of anhydrous oxalic acid in 80 mL of anhydrous dichloromethane under nitrogen protection conditions, slowly add 23 mL of triethylamine dropwise at a temperature lower than 10 ° C, stir and react for 30 minutes after the dropwise addition, and then Slowly add 80 mL of anhydrous dichloromethane containing 16.2 g (75 mmol) of p-nitrobenzyl bromide dropwise at a temperature of 10°C. After the dropwise addition, stir and react for 36 hours, filter with suction, and extract the filtrate with water (80 mL×5 times). The water extract was adjusted to pH 2.5 with 10% hydrochloric acid solution by mass percentage, and then extracted with ethyl acetate (80mL×5 times), the extract was washed with saturated ...
Embodiment 2
[0041] Embodiment two, the synthesis of thiamycin derivative
[0042] 1. (3S, 4R)-3-[(1R)-1-(tert-butyldimethylsilyloxy)ethyl]-4-{2-[2-(tert-butoxycarbonylamino)ethylthio Synthesis of ]carboxyethyl}-2-azetidinone-1-oxoacetic acid p-nitrobenzyl ester (V)
[0043] The preparation method of single p-nitrobenzyl oxalate is identical with the method described in embodiment one;
[0044] Calculate the amount of raw materials according to the theoretical production amount of 4.4g of the product; under the condition of nitrogen protection, dissolve 6g (26.7mmol) of mono-p-nitrobenzyl oxalate in 20mL (280.6mmol) of thionyl chloride, and reflux at a temperature of 55°C Reacted for 3.5 hours, cooled to room temperature, and distilled off the solvent under reduced pressure to obtain 6.5 g of mono-p-nitrobenzyl oxalyl chloride as a white solid, which was dissolved by adding 40 mL of anhydrous toluene and directly used in the next reaction; compound IV 3 g (6.7 mmol ) was dissolved in 30m...
Embodiment 3
[0048] Embodiment three, the synthesis of thiamycin derivative
[0049] 1. (3S, 4R)-3-[(1R)-1-(tert-butyldimethylsilyloxy)ethyl]-4-{2-[2-(tert-butoxycarbonylamino)ethylthio Synthesis of ]carboxyethyl}-2-azetidinone-1-oxoacetic acid p-nitrobenzyl ester (V)
[0050] The preparation method of single p-nitrobenzyl oxalate is identical with the method described in embodiment one;
[0051] Calculate the amount of raw materials according to the theoretical production amount of 4.4g of the product; under the condition of nitrogen protection, dissolve 4.5g (20.1mmol) of mono-p-nitrobenzyl oxalate in 20mL (280.6mmol) of thionyl chloride. Reflux for 3.5 hours, cool to room temperature, and distill off the solvent under reduced pressure to obtain 4.9 g of mono-p-nitrobenzyl oxalyl chloride as a white solid, which was dissolved by adding 40 mL of anhydrous toluene and directly used in the next reaction; 3 g of compound IV ( 6.7mmol) was dissolved in 30mL of anhydrous toluene, and slowly ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com