4AA intermediate refining method
A refining method and intermediate technology, applied in the field of chemical drug refining process, can solve the problems of increasing the amount of post-treatment reagents, increasing the consumption of raw materials, reducing the conversion rate, etc., and achieve the effects of avoiding side reactions, reducing the amount, and increasing the yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
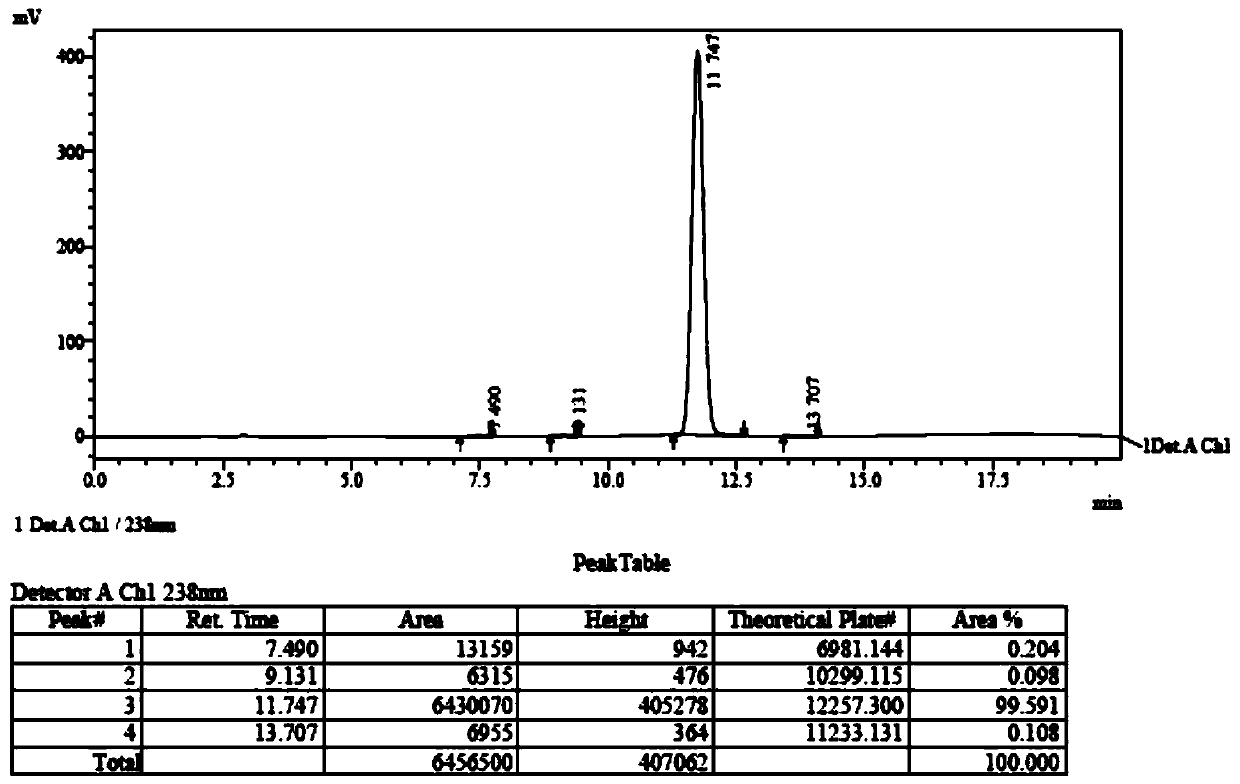
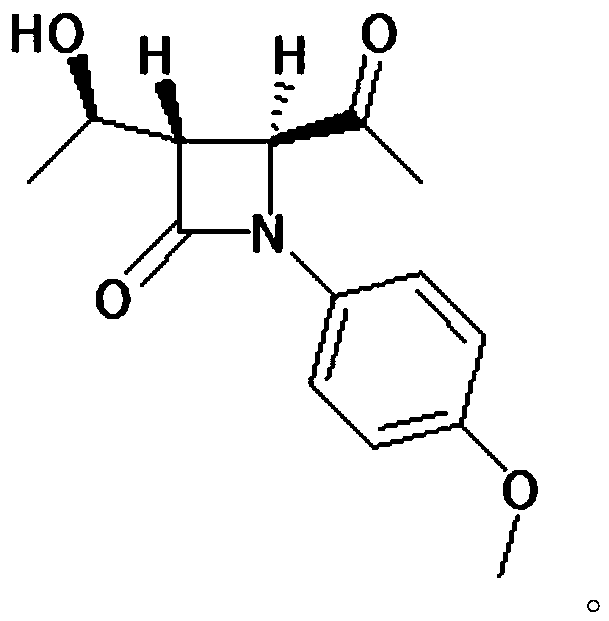
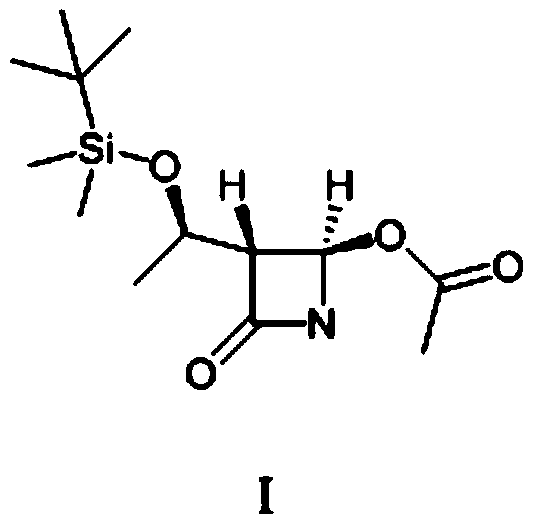
[0045] Add (52.7g, 0.20mol) (2R,3R)-N-(4-methoxyphenyl)-N-(2-oxopropyl)-2,3-epoxybutanamide to a 500ml four-necked bottle , 250g of dichloromethane and (89g, 0.88mol) of diisopropylamine, cooled to -30°C, slowly added dropwise (41.6g, 0.22mol) of titanium tetrachloride, the dropwise addition was completed, and kept for 0.5h. The reaction solution was dropped into dilute hydrochloric acid, stirred and allowed to stand for liquid separation, and the aqueous layer was extracted with 400 g of dichloromethane. The organic layers were combined, and the organic layer was washed with saturated sodium bicarbonate until neutral, and dichloromethane was evaporated from the organic phase under reduced pressure. Add 100 mL of diethyl ether and 100 mL of petroleum ether to 60 g of the concentrate (purity is about 80%), raise the temperature to 40° C., and stand to separate layers. This operation was repeated three times, the supernatants were combined, and crystallized at 0°C for 1 h, filt...
Embodiment 2
[0050] Add (52.7g, 0.20mol) (2R,3R)-N-(4-methoxyphenyl)-N-(2-oxopropyl)-2,3-epoxybutanamide to a 500ml four-necked bottle , 250g of dichloromethane and (89g, 0.88mol) of diisopropylamine, cooled to -30°C, slowly added dropwise (41.6g, 0.22mol) of titanium tetrachloride, the dropwise addition was completed, and kept for 0.5h. The reaction solution was dropped into dilute hydrochloric acid, stirred and allowed to stand for liquid separation, and the aqueous layer was extracted with 400 g of dichloromethane. The organic layers were combined, and the organic layer was washed with saturated sodium bicarbonate until neutral, and dichloromethane was evaporated from the organic phase under reduced pressure. Add 100 mL of diethyl ether and 100 mL of petroleum ether to 60 g of the concentrate (purity is about 80%), raise the temperature to 40° C., and stand to separate layers. This operation was repeated three times, the supernatants were combined, and crystallized at 5°C for 1 h, filt...
Embodiment 3
[0052] Add (52.7g, 0.20mol) (2R,3R)-N-(4-methoxyphenyl)-N-(2-oxopropyl)-2,3-epoxybutanamide to a 500ml four-necked bottle , 250g of dichloromethane and (89g, 0.88mol) of diisopropylamine, cooled to -30°C, slowly added dropwise (41.6g, 0.22mol) of titanium tetrachloride, the dropwise addition was completed, and kept for 0.5h. The reaction solution was dropped into dilute hydrochloric acid, stirred and allowed to stand for liquid separation, and the aqueous layer was extracted with 400 g of dichloromethane. The organic layers were combined, and the organic layer was washed with saturated sodium bicarbonate until neutral, and dichloromethane was evaporated from the organic phase under reduced pressure. Add 100 mL of diethyl ether and 100 mL of petroleum ether to 60 g of the concentrate (purity is about 80%), raise the temperature to 40° C., and stand to separate layers. This operation was repeated three times, the supernatants were combined, and crystallized at 10°C for 1 h, fil...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com