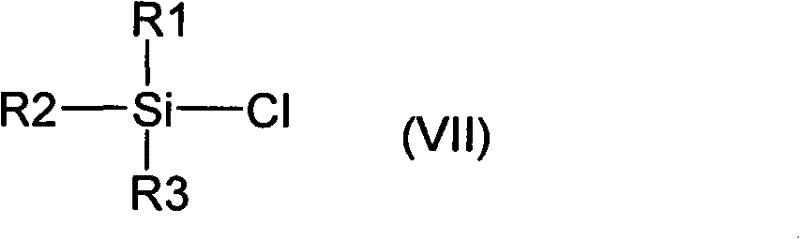Novel ezetimibe synthesis method
A technology of etimibe and a new method, which is applied in the field of synthesizing 1---[3---hydroxypropyl]---2-propiolactam, can solve the problem of limited application, low reaction yield and high reaction yield. Acid waste and other problems, to achieve the effect of convenient purification, high purity and little environmental pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
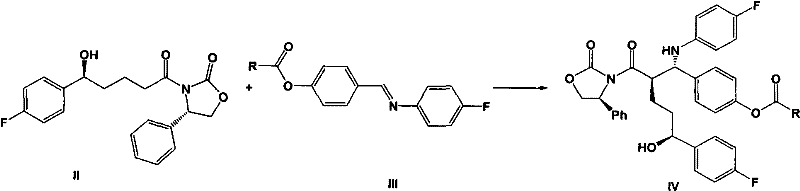
[0062] Example 1: 3-[(5-(4-fluorophenyl)-(5S)-hydroxypentanoyl]-3-{(2R)-[1-(4-acetoxyphenyl)-(1S) Synthesis of -(4-fluoroanilino)]-(4S)-phenyloxazolidinone (IVa)
[0063]
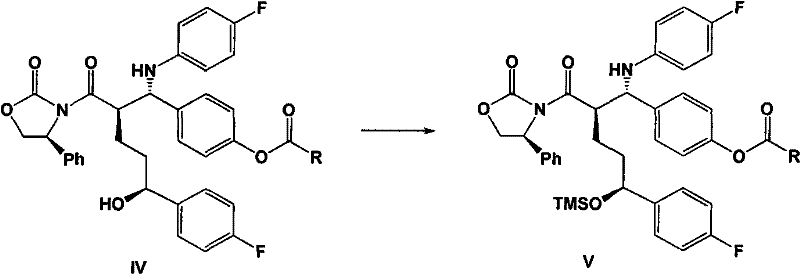
[0064] At room temperature, 30 grams of 3-[(5-(4-fluorophenyl)-(5S)-hydroxypentanoyl]-(4S)-phenyloxazolidinone (II), 22 Gram N-4'-acetoxybenzylidene-4-fluoroaniline (IIIa), 30 milliliters of triethylamine and 600 milliliters of dichloromethane. Add 10 ml of trimethylchlorosilane dropwise, and stir at 25°C for 20 hours after the dropwise addition. Cool down to 10°C, and add 15 ml of titanium tetrachloride solution dropwise to the system within 30 minutes. Stir under low temperature for 12 hours. After the reaction, the internal temperature does not exceed 15°C, slowly add 1 liter of 1N hydrochloric acid aqueous solution dropwise to the system, then add 600 ml of dichloromethane, and extract the organic phase. The organic phase is dried over anhydrous sodium sulfate Finally, the organic solvent was remov...
Embodiment 2
[0065] Example 2: 1-(4-fluorophenyl)-(3R)-[3-(4-fluorophenyl)-(3S)-hydroxypropyl]-(4S)-(4-hydroxyphenyl)- Synthesis of 2-propiolactam (ezetimibe, I)
[0066]
[0067] Add 23 grams of 3-[(5-(4-fluorophenyl)-(5S)-hydroxypentanoyl]-3-{(2R)-[1-(4-acetoxyphenyl)- (1S)-(4-fluoroanilino)]-(4S)-phenyloxazolidinone (IVa), 350 milliliters of toluene and 18.5 milliliters of BSA, followed by reflux for 6 hours. Then add in batches within 1 hour 2 grams of tetra-n-butylammonium fluoride, keep this temperature and continue to react for 6 hours. Cool down to room temperature, add 1 liter of water to this system, and under vigorous stirring, slowly add 55 milliliters of 2N sulfuric acid dropwise, and stir for 2 hours, the solid precipitates 。 Filtrate, dry the solid, and recrystallize in toluene to obtain 12.6 g of white solid with a yield of 82% and a purity of 99.6%.
[0068] Reference Example 1: Synthesis of N-4'-acetoxybenzylidene-4-fluoroaniline (IIIa)
[0069] Add 144 grams of p-h...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com