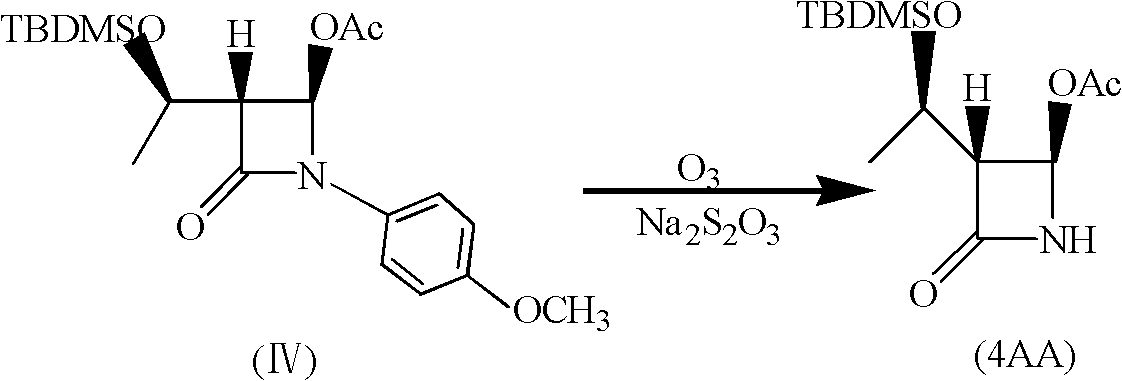
Synthesis method of 4-acetoxyl-2-azetidinone
The technology of azetidinone and acetoxy is applied in the field of synthesis of penem drug intermediate 4-acetoxy-2-azetidinone, which can solve the problem of high cost of raw materials, large environmental pollution and high cost high problems, to overcome the high cost of raw materials, shorten the reaction cycle, and achieve the effect of less pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
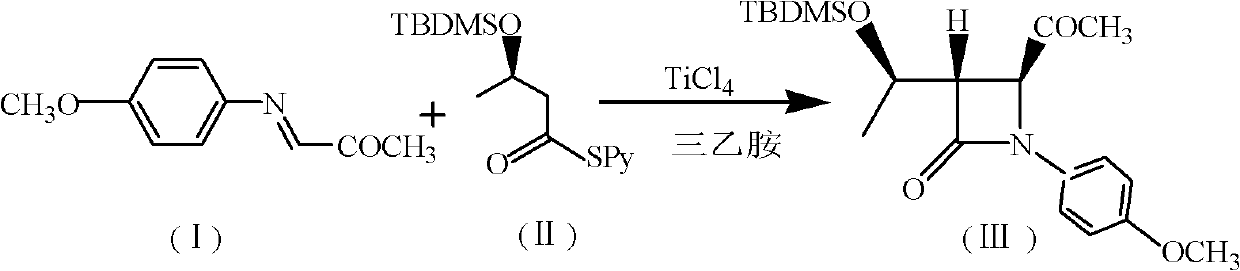
[0044] (1) Add 200mL chloroform, 37.4g (0.12mol) (R) 3-tert-butyldimethylsilyloxy-thiobutyric acid-S-2-pyridyl ester, 30mL triethylamine to 500mL tetra In the mouth bottle, cool down to -10~-20°C, add 19g TiCl dropwise under the protection of nitrogen 4 , and then added 17.7g (0.1mol) of N-p-methoxyphenyl-N-(acetyl)methylimine, and reacted at 0-5°C for 5h. After the reaction, the nitrogen protection was stopped, followed by 50mL 1N NaOH, 80mL saturated NaHCO 3 solution and washed with 100 mL of water. The organic layer was dried over anhydrous sodium sulfate, filtered, and the filtrate was evaporated to dryness to obtain (3S, 4S)-3-[(1'R)-tert-butyldimethylsiloxyethyl]-4-acetyl-1- p-methoxyphenyl-2-azetidinone 27.6g, yield 73.4%, mp: 81-83°C, purity: 98.8% (HPLC detection).
[0045]
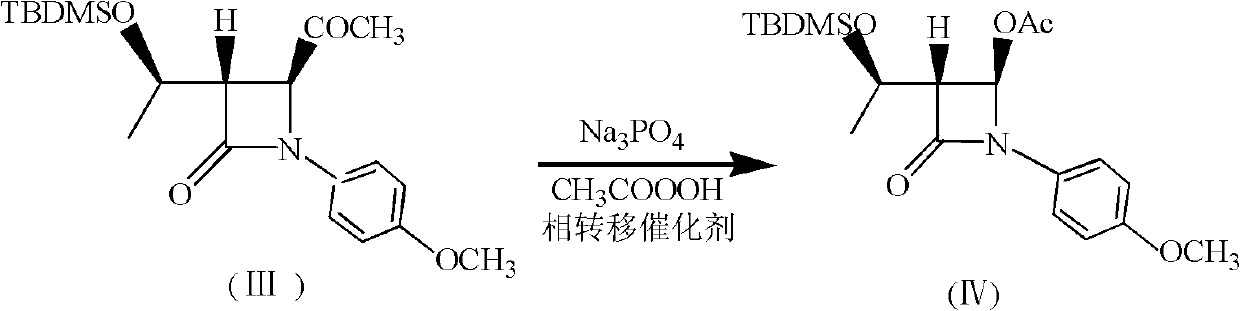
[0046] (2) Add 150mL ethyl acetate to a 500mL three-necked flask, and 27.6g (0.073mol) (3S, 4S)-3-[(1'R)-tert-butyldimethylsiloxyethyl]-4- Acetyl-1-p-methoxyphenyl-2-azetidinone was dissol...
Embodiment 2
[0051] (1) Add 200mL chloroform, 46.7g (0.15mol) (R) 3-tert-butyldimethylsilyloxy-thiobutyric acid-S-2-pyridyl ester, 30mL triethylamine to 500mL tetra In the mouth bottle, cool down to -10~-20°C, add 19g TiCl dropwise under the protection of nitrogen 4 , add 17.7g (0.1mol) of N-p-methoxyphenyl-N-(acetyl)methylimine, react at 0~5℃ for 5h. After the reaction, the nitrogen protection was stopped, followed by 50mL 1N NaOH, 80mL saturated NaHCO 3 solution and washed with 100 mL of water. The organic layer was dried over anhydrous sodium sulfate, filtered, and the filtrate was evaporated to dryness to obtain (3S, 4S)-3-[(1'R)-tert-butyldimethylsiloxyethyl]-4-acetyl-1- p-methoxyphenyl-2-azetidinone 28.9g, yield 76.8% (based on compound I, yield calculation formula is the same as Example 1), mp: 81-83°C, purity: 98.6% (HPLC detection).
[0052] (2) Add 150mL ethyl acetate to a 500mL three-necked flask, and 28.9g (0.077mol) (3S, 4S)-3-[(1'R)-tert-butyldimethylsiloxyethyl]-4- Diss...
Embodiment 3
[0055] (1) Add 200mL chloroform, 40.5g (0.13mol) (R) 3-tert-butyldimethylsilyloxy-thiobutyric acid-S-2-pyridyl ester, 30mL triethylamine to 500mL tetra In the mouth bottle, cool down to -10~-20°C, add 19g TiCl dropwise under the protection of nitrogen 4 , add 17.7g (0.1mol) of N-p-methoxyphenyl-N-(acetyl)methylimine, react at 0~5℃ for 5h. After the reaction, the nitrogen protection was stopped, followed by 50mL 1N NaOH, 80mL saturated NaHCO 3 solution and washed with 100 mL of water. The organic layer was dried over anhydrous sodium sulfate, filtered, and the filtrate was evaporated to dryness to obtain (3S, 4S)-3-[(1'R)-tert-butyldimethylsiloxyethyl]-4-acetyl-1- p-Methoxyphenyl-2-azetidinone 28.4g, yield 75.5% (based on compound I, yield calculation formula is the same as Example 1), mp: 81-83°C, purity: 98.5% (HPLC detection).
[0056] (2) Add 150mL ethyl acetate to a 500mL three-necked flask, and 28.4g (0.075mol) (3S, 4S)-3-[(1'R)-tert-butyldimethylsiloxyethyl]-4- Diss...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com