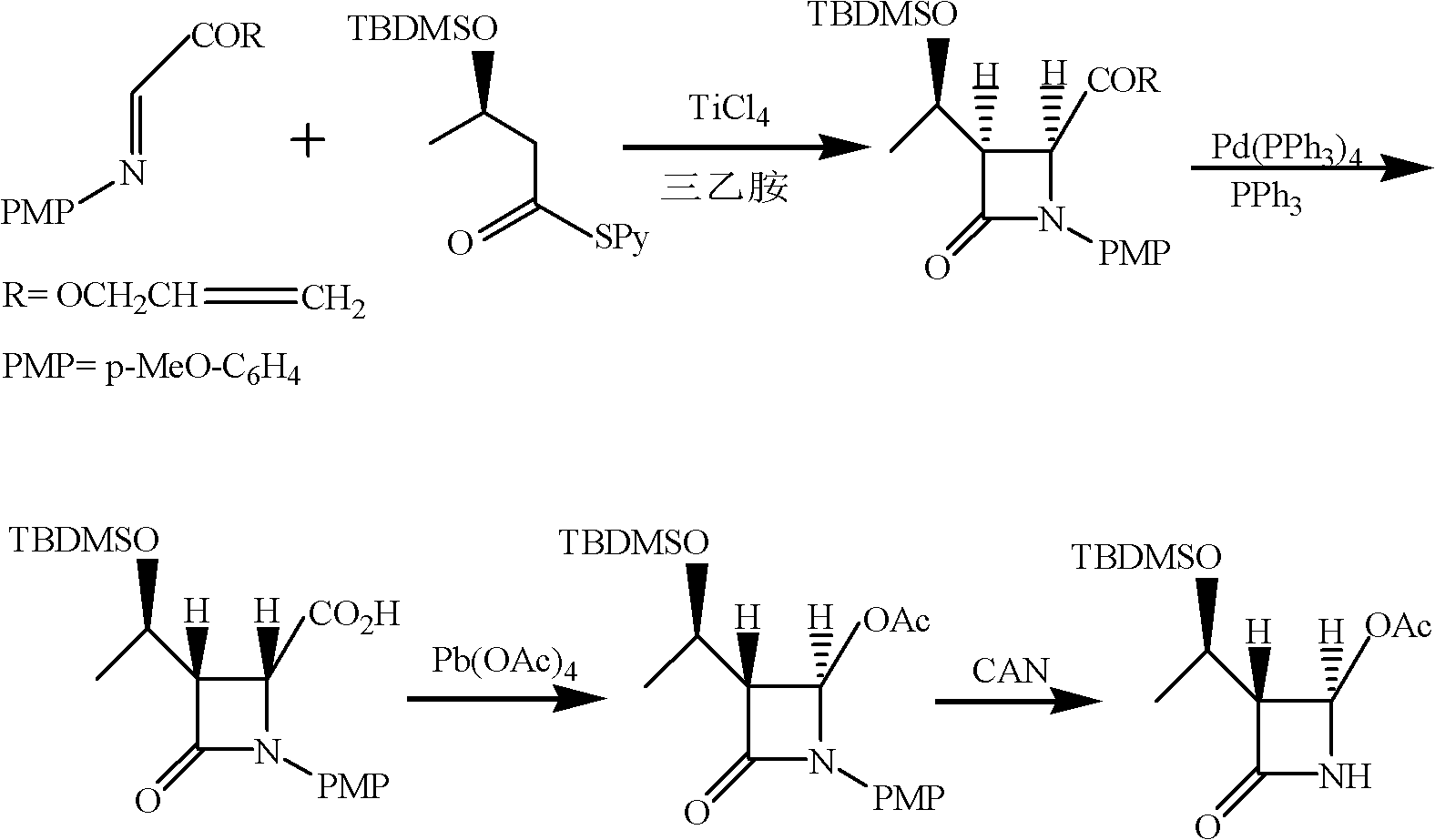
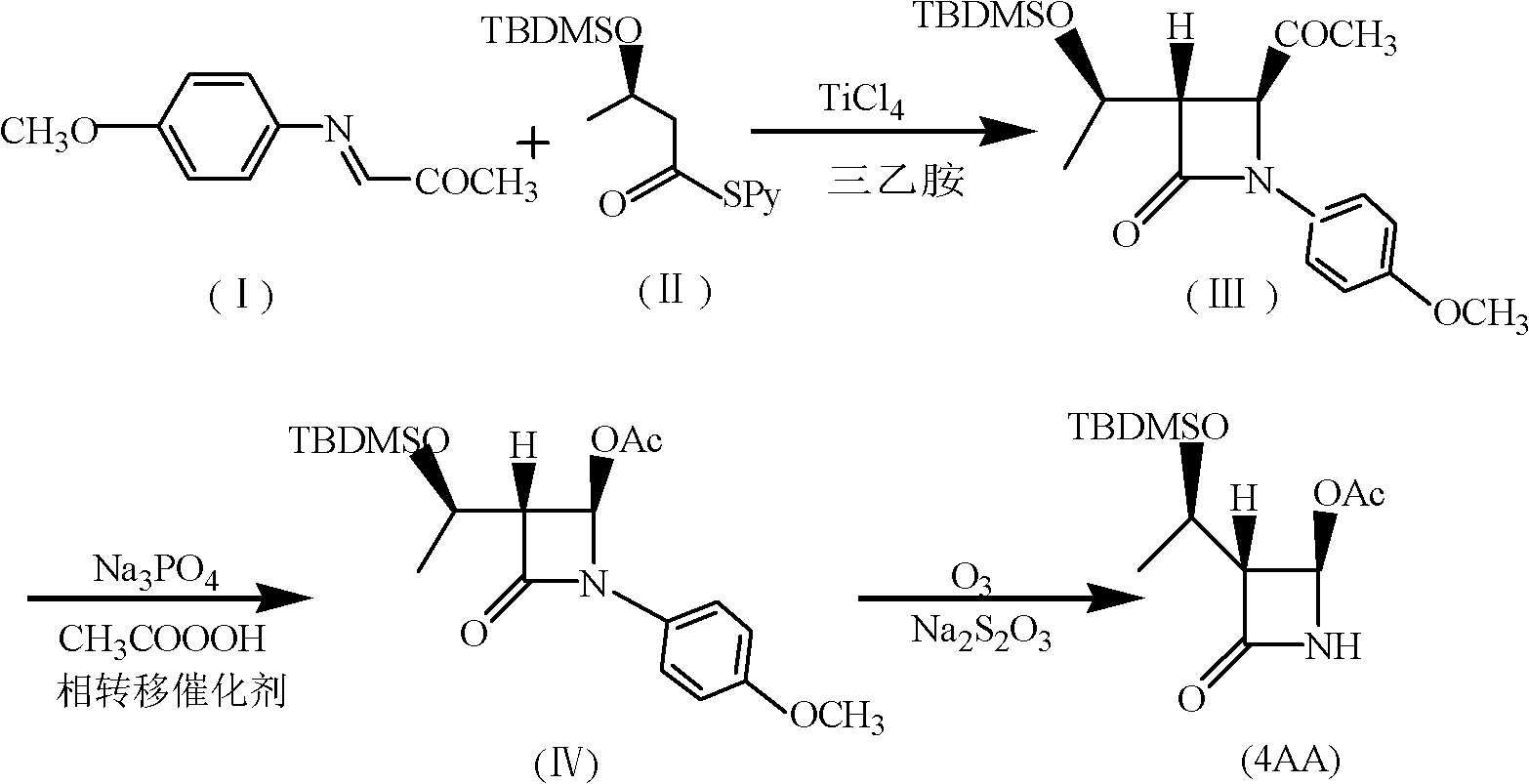
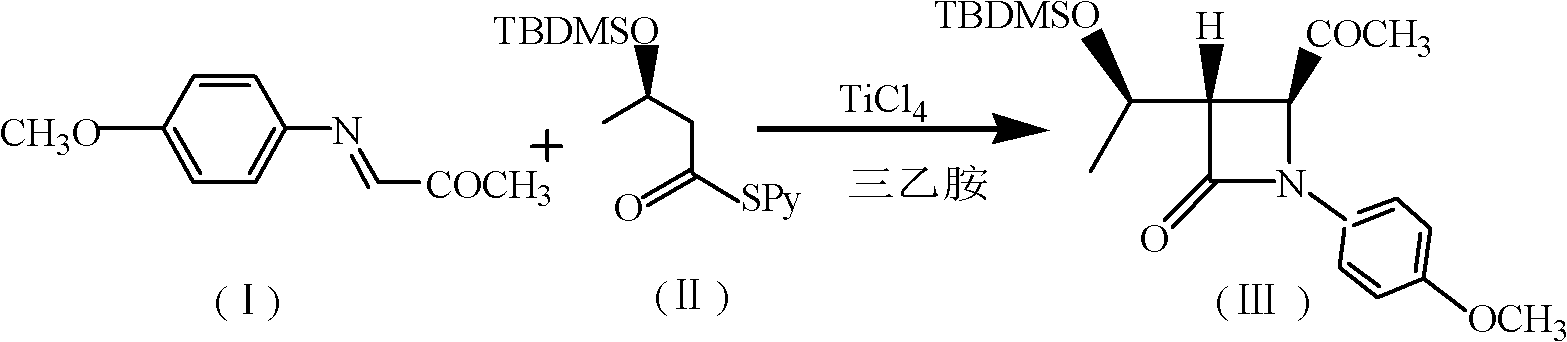Synthesis method of 4-acetoxyl-2-azetidinone
A technology of -acetoxy- and azetidinone, which is applied in the field of synthesis of penem drug intermediate 4-acetoxy-2-azetidinone, can solve the problem of high cost of raw materials and large environmental pollution , the problem of high cost, to overcome the high cost of raw materials, shorten the reaction cycle, and achieve the effect of low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] (1) Add 200mL chloroform, 37.4g (0.12mol) (R) 3-tert-butyldimethylsilyloxy-thiobutyric acid-S-2-pyridyl ester, 30mL triethylamine to 500mL tetra In the mouth bottle, cool down to -10~-20°C, add 19g TiCl dropwise under the protection of nitrogen 4 , and then added 17.7g (0.1mol) of N-p-methoxyphenyl-N-(acetyl)methylimine, and reacted at 0-5°C for 5h. After the reaction, the nitrogen protection was stopped, followed by 50mL 1N NaOH, 80mL saturated NaHCO 3 solution and washed with 100 mL of water. The organic layer was dried over anhydrous sodium sulfate, filtered, and the filtrate was evaporated to dryness to obtain (3S, 4S)-3-[(1'R)-tert-butyldimethylsiloxyethyl]-4-acetyl-1- p-methoxyphenyl-2-azetidinone 27.6g, yield 73.4%, mp: 81-83°C, purity: 98.8% (HPLC detection).
[0045]
[0046] (2) Add 150mL ethyl acetate to a 500mL three-necked flask, and 27.6g (0.073mol) (3S, 4S)-3-[(1'R)-tert-butyldimethylsiloxyethyl]-4- Acetyl-1-p-methoxyphenyl-2-azetidinone was dissol...
Embodiment 2
[0051] (1) Add 200mL chloroform, 46.7g (0.15mol) (R) 3-tert-butyldimethylsilyloxy-thiobutyric acid-S-2-pyridyl ester, 30mL triethylamine to 500mL tetra In the mouth bottle, cool down to -10~-20°C, add 19g TiCl dropwise under the protection of nitrogen 4 , add 17.7g (0.1mol) of N-p-methoxyphenyl-N-(acetyl)methylimine, react at 0~5℃ for 5h. After the reaction, the nitrogen protection was stopped, followed by 50mL 1N NaOH, 80mL saturated NaHCO 3 solution and washed with 100 mL of water. The organic layer was dried over anhydrous sodium sulfate, filtered, and the filtrate was evaporated to dryness to obtain (3S, 4S)-3-[(1'R)-tert-butyldimethylsiloxyethyl]-4-acetyl-1- p-methoxyphenyl-2-azetidinone 28.9g, yield 76.8% (based on compound I, yield calculation formula is the same as Example 1), mp: 81-83°C, purity: 98.6% (HPLC detection).
[0052] (2) Add 150mL ethyl acetate to a 500mL three-necked flask, and 28.9g (0.077mol) (3S, 4S)-3-[(1'R)-tert-butyldimethylsiloxyethyl]-4- Diss...
Embodiment 3
[0055] (1) Add 200mL chloroform, 40.5g (0.13mol) (R) 3-tert-butyldimethylsilyloxy-thiobutyric acid-S-2-pyridyl ester, 30mL triethylamine to 500mL tetra In the mouth bottle, cool down to -10~-20°C, add 19g TiCl dropwise under the protection of nitrogen 4 , add 17.7g (0.1mol) of N-p-methoxyphenyl-N-(acetyl)methylimine, react at 0~5℃ for 5h. After the reaction, the nitrogen protection was stopped, followed by 50mL 1N NaOH, 80mL saturated NaHCO 3 solution and washed with 100 mL of water. The organic layer was dried over anhydrous sodium sulfate, filtered, and the filtrate was evaporated to dryness to obtain (3S, 4S)-3-[(1'R)-tert-butyldimethylsiloxyethyl]-4-acetyl-1- p-Methoxyphenyl-2-azetidinone 28.4g, yield 75.5% (based on compound I, yield calculation formula is the same as Example 1), mp: 81-83°C, purity: 98.5% (HPLC detection).
[0056] (2) Add 150mL ethyl acetate to a 500mL three-necked flask, and 28.4g (0.075mol) (3S, 4S)-3-[(1'R)-tert-butyldimethylsiloxyethyl]-4- Diss...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com