Method for synthesizing ezetimibe
A technology of ezetimibe and compounds, which is applied in the field of ezetimibe synthesis, can solve the problems of large-scale industrial production and high production cost, harsh reaction conditions, and complex operation in the process, so as to avoid the use of chiral reagents , Reduction of reaction steps, beneficial to the effect of industrial production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
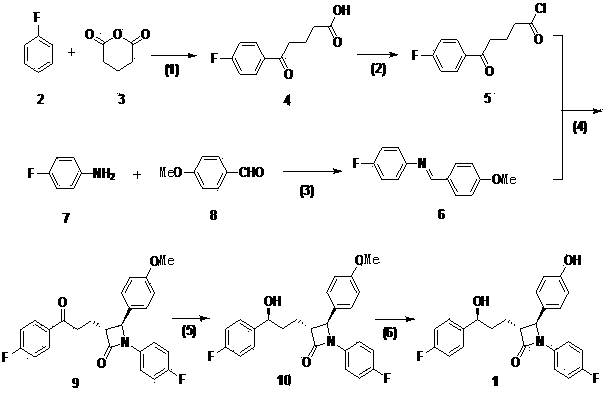
[0041] (1) Add 0.72g of aluminum trichloride (0.0054mol) to 0.90mL (0.0092mol) of fluorobenzene and mix well, add glutaric anhydride (0.3g, 0.0025mol) and fluorobenzene (1.1ml, 0.012 mol) of the mixed solution, after dropping, react at room temperature for 5 hours. After the reaction, cool in an ice bath, add 1.5mL of 1mol / L hydrochloric acid dropwise, then pour into ice water, a large amount of white solid precipitates, filter, wash the filter cake with ice water, then put the filter cake into 8mL of 10% sodium hydroxide React in the solution at 60°C for 1 hour, filter while hot, adjust the pH value of the filtrate to 1 with hydrochloric acid, place the solution at 0°C for 1-2 hours, filter, heat the filter cake with ethyl acetate to dissolve, filter while hot, and spin the filtrate to dry A white solid was obtained, which was dried to yield 0.7 g of compound 4 as a white solid.
[0042](2) Dissolve 0.7 g of compound 4 in dichloromethane, reflux at 50°C, slowly add 25 mL of ...
Embodiment 2
[0049] (1) Add 3.6g of aluminum trichloride (0.027mol) into 4.5mL (0.046mol) of fluorobenzene and mix well, add glutaric anhydride (1.5g, 0.0125mol) and fluorobenzene (5.5ml, 0.06 mol) of the mixed solution, after dropping, react at room temperature for 5 hours. Cool in an ice bath after the reaction, add 10mL of 1mol / L hydrochloric acid dropwise, then pour into ice water, a large amount of white solid precipitates, filter, wash the filter cake with ice water, then put the filter cake into 50mL of 10% sodium hydroxide solution React at 60°C for 1 hour, filter while hot, adjust the pH value of the filtrate to 1 with hydrochloric acid, place the solution at 0°C for 1 to 2 hours, filter, heat the filter cake with ethyl acetate to dissolve, filter while hot, and spin the filtrate to obtain The white solid was dried to obtain 3.06 g of compound 4 as a white solid.
[0050] (2) Dissolve 3.06g of compound 4 in dichloromethane, reflux at 70°C, slowly add 2.35mL of thionyl chloride dr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com