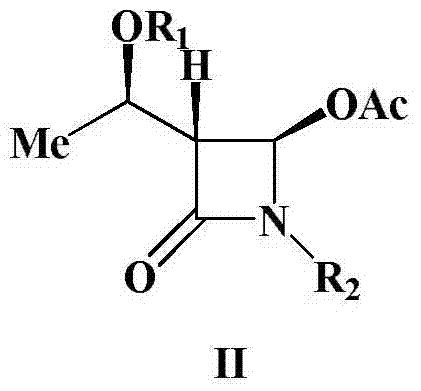
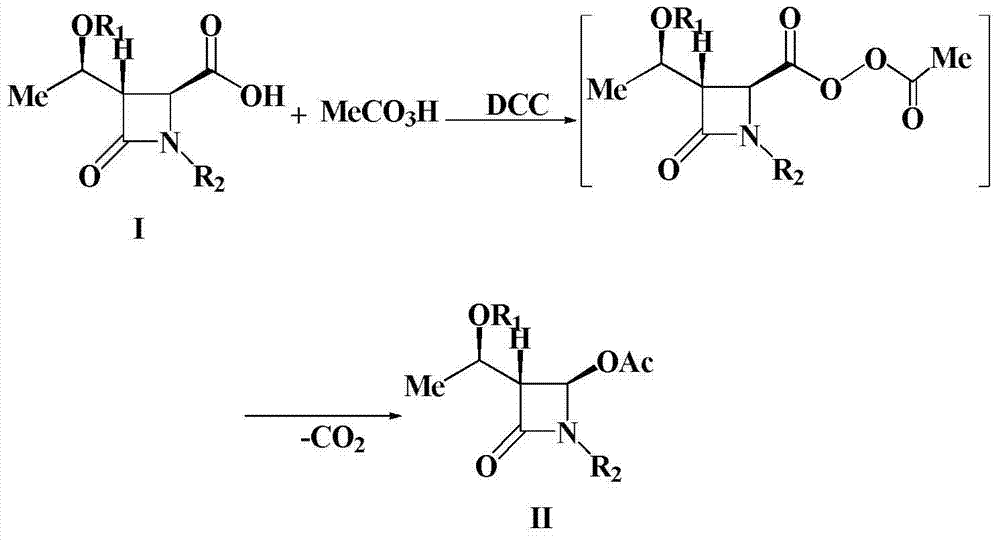
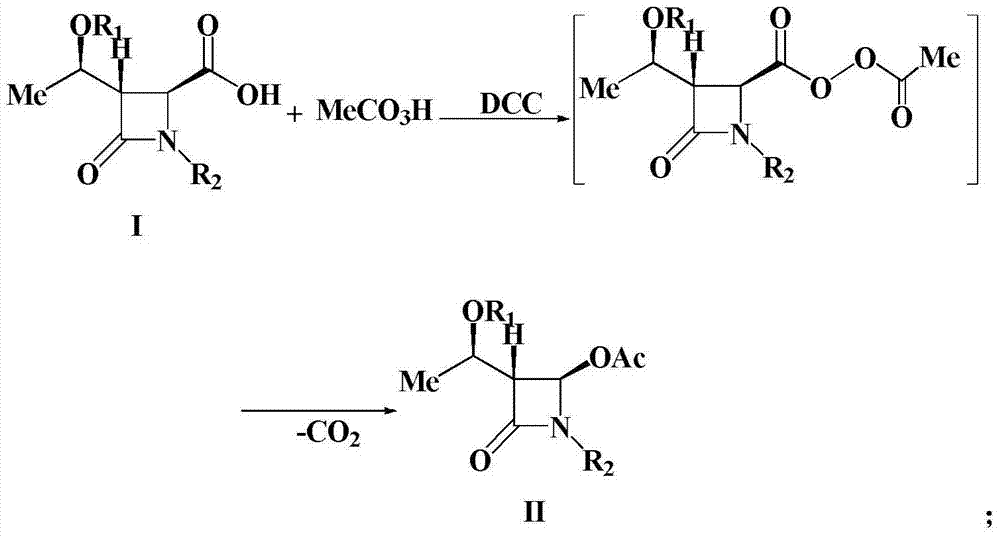
Preparation method of 4-acetyloxy-2-azetidinone compounds
A technology of azetidinone and acetoxy, which is applied in the fields of compounds of group 4/14 elements of the periodic table, chemical instruments and methods, organic chemistry, etc., can solve unfavorable large-scale production, harsh reaction conditions, and reaction condition requirements Harshness and other problems, to achieve the effect of convenient recovery and reuse, mild reaction conditions, easy separation and purification
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Example 1. (3S,4S)-3-[(R)-1-tert-butyldimethylsiloxyethyl]-4-acetoxy-1-(4-methoxyphenyl)-2 - Synthesis of azetidinone
[0025] Add (3S,4S)-3-[(R)-1-tert-butyldimethylsiloxyethyl]-4-carboxy-1-(4-methoxyphenyl) sequentially into a 500mL three-neck flask -2-azetidinone 37.9g (0.1mol), dichloromethane (200mL), placed under ice bath conditions, then slowly added 23.9g35% peracetic acid (0.11mol), after the peracetic acid was added , and then add 22.7g (0.11mol) of N,N'-dicyclohexylcarbodiimide (DCC), and keep the reaction at 0-5°C for 1-2h. TLC monitors the reaction. After the reaction is over, the reaction mixture is first filtered, and the filtrate is washed with 300 mL of water each time for a total of 3 times. The organic layer obtained after washing with water is washed with saturated NaHCO 3 Wash, anhydrous Na 2 SO 4 After drying, the solvent was evaporated under reduced pressure, and the crude product was recrystallized to obtain 37.7g of a white solid product. ...
Embodiment 2
[0026] Example 2. (3S,4S)-3-[(R)-1-tert-butyldimethylsiloxyethyl]-4-acetoxy-1-(4-methoxyphenyl)-2 - Synthesis of azetidinone
[0027] Add (3S,4S)-3-[(R)-1-tert-butyldimethylsiloxyethyl]-4-carboxy-1-(4-methoxyphenyl) sequentially into a 500mL three-neck flask - 37.9g (0.1mol) of 2-azetidinone, chloroform (150mL), placed in an ice bath, then slowly added 49.4g of 20% peroxyacetic acid (0.13mol), after the peracetic acid was added, then Add 26.8g (0.13mol) of N,N'-dicyclohexylcarbodiimide (DCC), and keep the reaction at 25°C for 1-2h. TLC monitors the reaction. After the reaction is over, the reaction mixture is first filtered, and the filtrate is washed with 300 mL of water each time for a total of 3 times. The organic layer obtained after washing with water is washed with saturated NaHCO 3 Wash, anhydrous Na 2 SO 4 After drying, the solvent was evaporated under reduced pressure, and the crude product was recrystallized to obtain 37.0 g of a white solid product. The struct...
Embodiment 3
[0028] Example 3. (3S,4S)-3-[(R)-1-tert-butyldimethylsiloxyethyl]-4-acetoxy-1-(4-methoxybenzyl)-2 - Synthesis of azetidinone
[0029]Add (3S,4S)-3-[(R)-1-tert-butyldimethylsiloxyethyl]-4-carboxy-1-(4-methoxybenzyl) sequentially into a 500mL three-neck flask -2-azetidinone 39.3g (0.1mol), dichloromethane (200mL), placed under ice bath conditions, then slowly added 26.1g35% peracetic acid (0.12mol), after the peracetic acid was added , and then add 24.8g (0.12mol) of N,N'-dicyclohexylcarbodiimide (DCC), and keep the reaction at 15°C for 1-2h. TLC monitors the reaction. After the reaction is over, the reaction mixture is first filtered, and the filtrate is washed with 300 mL of water each time for a total of 3 times. The organic layer obtained after washing with water is washed with saturated NaHCO 3 Wash, anhydrous Na 2 SO 4 After drying, the solvent was evaporated under reduced pressure, and the crude product was recrystallized to obtain 36.6 g of a white solid product. The...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com