Method for reduction preparation of 4-AA with inorganic reducing agent
A reducing agent and inorganic technology, applied in the field of β-lactam drugs, can solve the problems of environmental protection pressure, large amount of wastewater discharge, and unknown mechanism, achieve significant environmental protection and economic value, reduce wastewater treatment costs, and avoid large-scale The effect of transformation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
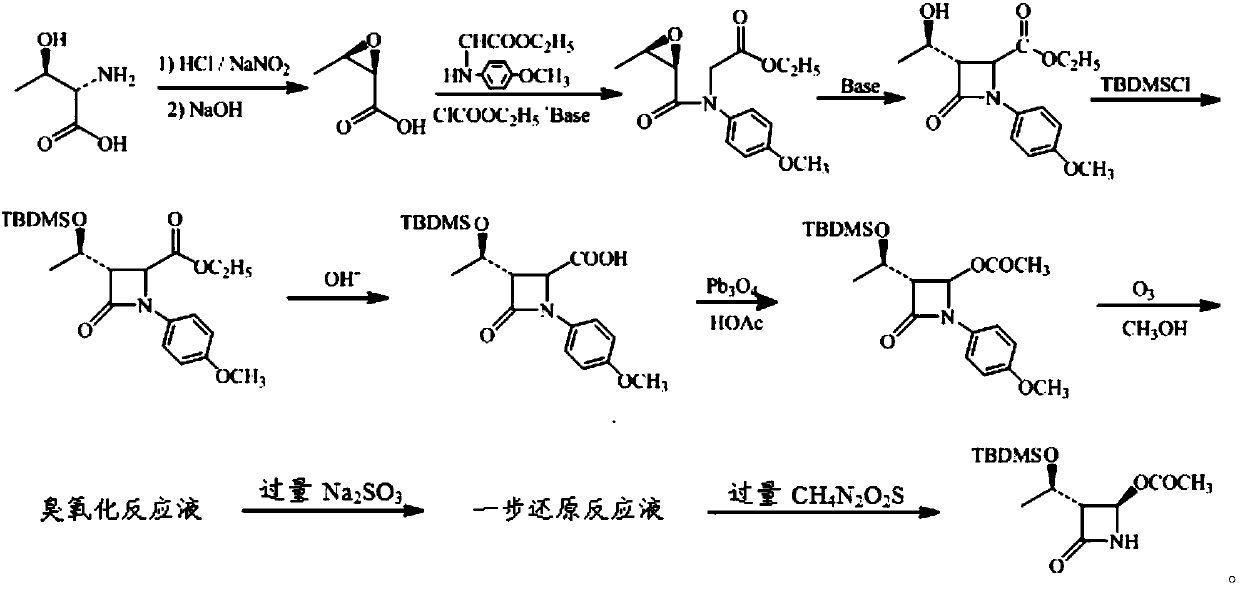
[0036] (1) Put 100g of zinc powder with a mesh number of 100 in 0.5mol / L NH 4 Activate in Cl aqueous solution, stir at room temperature for 2 hours, filter, wash with distilled water until it becomes neutral, and vacuum-dry at 70°C under reduced pressure to obtain activated zinc powder for future use;
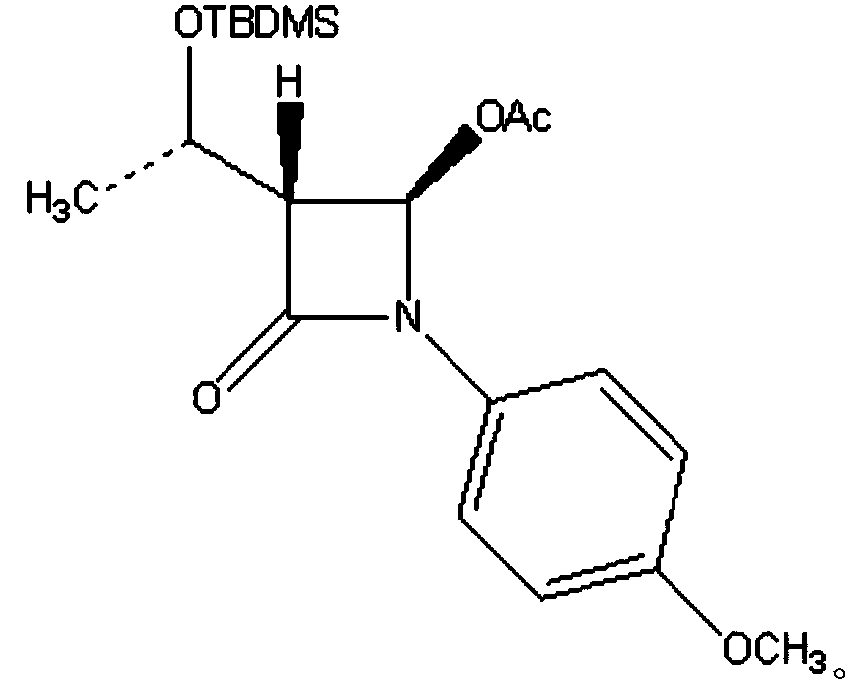
[0037] (2) Add 20.0 grams of compound I to a 250ml three-necked flask, add 150ml of methanol, stir at room temperature until clear (that is, the solution of compound I is obtained), the temperature of the system drops to -20°C, and ozone is introduced to react, and the temperature is maintained at - Between 20--15°C, TLC detects the reaction until the raw material point disappears (that is, the ozonated reaction liquid is obtained), slowly adds 35g of activated zinc powder obtained in step (1) to carry out the reduction reaction, slowly raises the temperature to 0°C, and reacts for 1.5 hours, the mixture was obtained;
[0038] (3) Filter the mixed solution obtained in step (2)...
Embodiment 2
[0042] (1) Place 100g of 80-mesh iron powder in 0.5mol / L HAc aqueous solution for activation, stir at 50°C for 2 hours, filter, wash with distilled water until it becomes neutral, and vacuum-dry at 70°C under reduced pressure to obtain activated iron powder, spare;
[0043] (2) Add 20.0 grams of compound I to a 250ml three-necked flask, add 150ml of methanol, stir at room temperature until clear (that is, the solution of compound I is obtained), the temperature of the system drops to -20°C, and ozone is introduced to react, and the temperature is maintained at - Between 20--15°C, TLC detects the reaction until the raw material point disappears (that is, the ozonated reaction solution is obtained), slowly add 23g of activated iron powder obtained in step (1) to carry out the reduction reaction, slowly raise the temperature to 0°C, and react for 2.5 hours, the mixture was obtained;
[0044] (3) Filter the mixed solution obtained in step (2), wash the filter cake with a small am...
Embodiment 3
[0048] (1) Put 80g of 100 mesh aluminum powder in 0.5mol / L NH 3 ·H 2 Activate in aqueous solution of O, stir at 50°C for 2 hours, filter, wash with distilled water until neutral, and vacuum-dry at 70°C under reduced pressure to obtain activated aluminum powder, which is ready for use;
[0049] (2) Add 20.0 grams of compound I to a 250ml three-necked flask, add 150ml of methanol, stir at room temperature until clear (that is, the solution of compound I is obtained), the temperature of the system drops to -20°C, and ozone is introduced to react, and the temperature is maintained at - Between 20--15°C, TLC detects the reaction until the raw material point disappears (that is, the ozonated reaction solution is obtained), slowly add 20g of the activated aluminum powder obtained in step (1) to carry out the reduction reaction, slowly raise the temperature to 0°C, and react for 1.5 hours, the mixture was obtained;
[0050] (3) Filter the mixed solution obtained in step (2), wash th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com