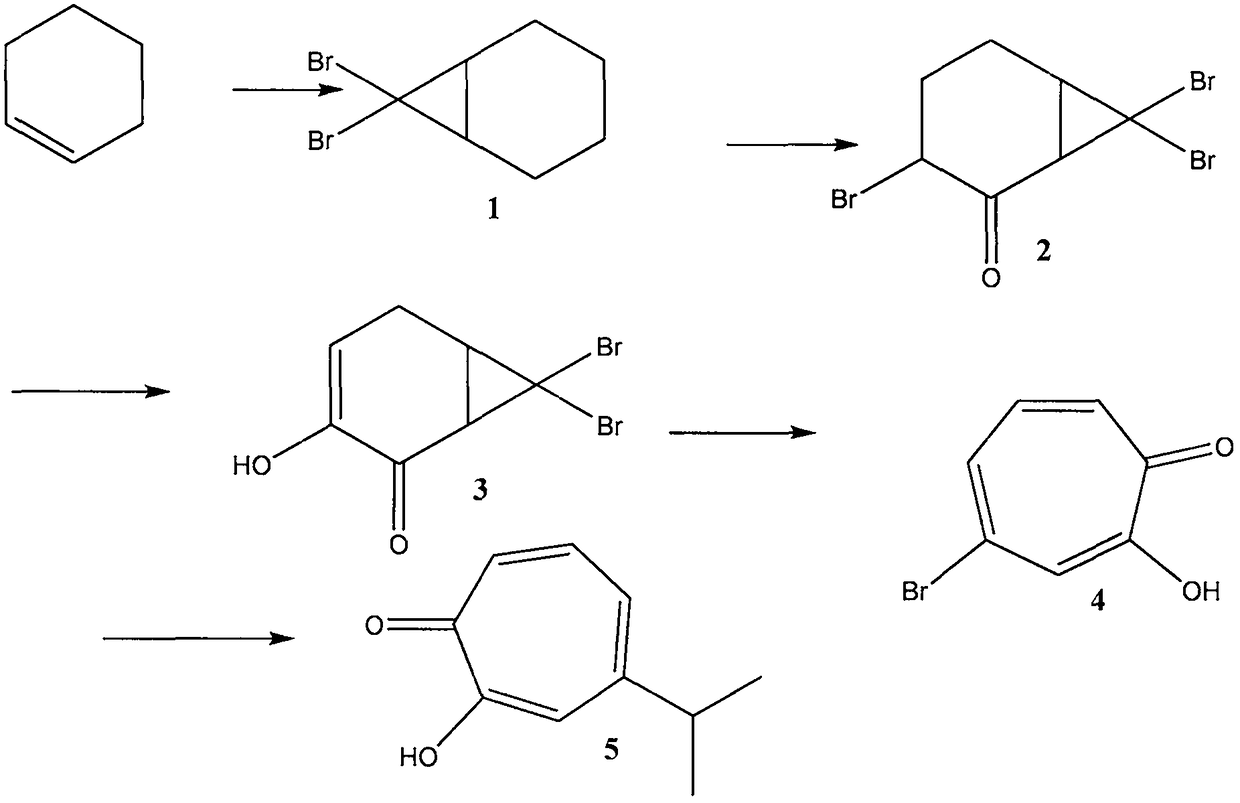
Method for preparing hinokitiol
A technology of asphalt alcohol and ethanol, which is applied in the field of industrialization and high-purity preparation, can solve the problems of strict equipment process requirements, high price, and easy formation of dimers, so as to avoid isomers and solve safety problems Problems and the effect of eliminating potential safety hazards
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
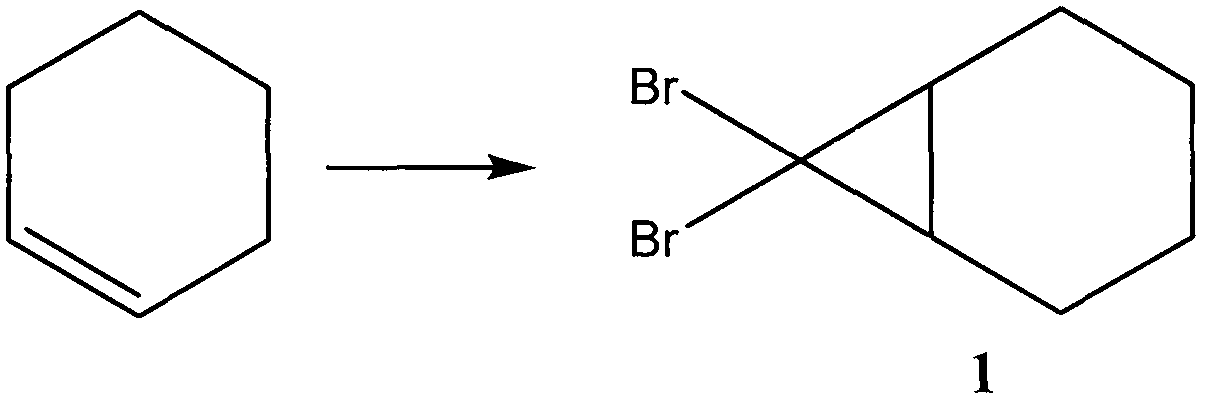
[0045] In the method for preparing cypress alcohol of the present invention, the preparation of compound 1 comprises the following steps:
[0046] The cyclohexene is mixed with bromomethane, and an addition reaction is carried out under the catalysis of triethylphenylammonium chloride and an inorganic base, the reaction temperature is -20 DEG C to 30 DEG C, and the reaction time is 0.5 to 30 hours; The volume ratio of hexene to tribromomethane is 1:0.5 to 1:100, preferably 1:2 to 1:10, and the volume ratio of cyclohexene to triethylphenylammonium chloride is 1000:1 to 10:1 , preferably 100:1 to 50:1; the inorganic base includes any one of sodium hydroxide and potassium hydroxide;
[0047] After the reaction was completed, it was quenched with hydrochloric acid, and the concentration of hydrochloric acid was 1M-10M; then, it was extracted and separated with dichloromethane, and compound 1 was collected from the dichloromethane solution, and the yield was 73%.
[0048] The gene...
Embodiment 1
[0049] Example 1 Synthetic preparation of compound 1
[0050] Dissolve 10 ml of cyclohexene in 50 ml of tribromomethane, add 0.1 g of triethylphenylammonium chloride (phase transfer catalyst), and cool the above solution to 0°C; after rapid stirring, add dropwise 24 g of 50% hydrogen Aqueous sodium oxide solution (nucleophilic reaction catalyst) was stirred at 0 °C for 2 hours, and then continued to stir at room temperature for 22 hours; then cooled to 0 °C, and 30 ml of 6M hydrochloric acid was added dropwise; the organic phase was separated, and the residual residue in the aqueous phase Compound 1 was extracted with dichloromethane; the organic phase was dried over anhydrous magnesium sulfate to remove desiccant and solvent; a colorless liquid compound 1 was obtained by distillation under reduced pressure with a yield of 73%.
[0051] The structure of compound 1 was characterized by hydrogen nuclear magnetic resonance spectroscopy (HNMR) and mass spectrometry (ESI-MS). The r...
Embodiment 2
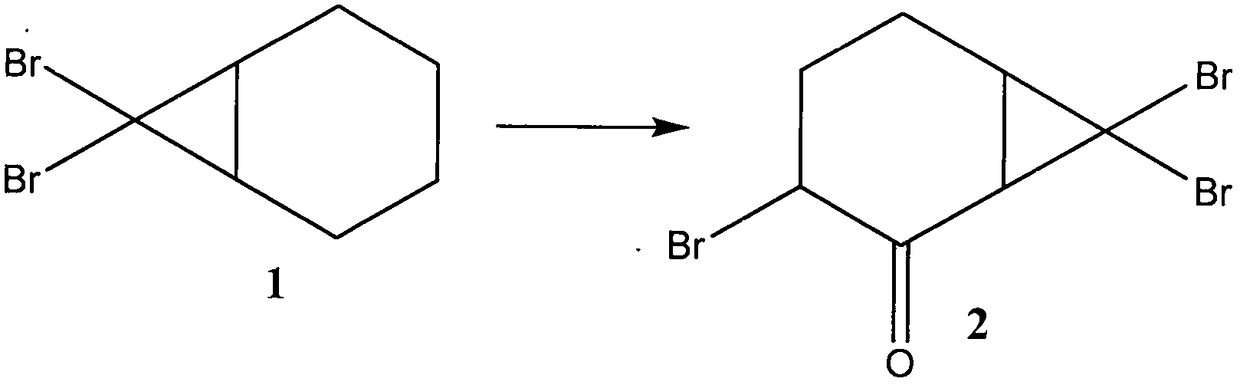
[0057] Example 2 Synthetic preparation of compound 2
[0058] 7.6 g of compound 1 was added to 200 ml of a solution of chromium trioxide (30 g) in ether at 0 °C, and stirred at 25-30 °C for 1 hour, the reaction was poured into 1 liter of water, the organic phase was separated, and the water The compound 2 remaining in the phase was extracted with ether; the separated organic phase and the extract were mixed, and washed with sodium carbonate aqueous solution and saturated brine to remove the impurities remaining in the organic phase; after drying with anhydrous magnesium sulfate, distilled off ether to give compound 2 (6.8 g, 90% yield).
[0059] The structure of compound 2 was characterized by hydrogen nuclear magnetic resonance spectroscopy and mass spectrometry, and the relevant data are as follows:
[0060] 1 H NMR (CDCl 3 , 300MHz), δ: 4.32 (t, 1H), 4.08 (d, 1H), 3.91 (m, 1H), 2.42 (m, 2H), 1.96 (m, 2H); ESI-MS: 343 (M + ).
[0061] In the method for preparing cypress...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com