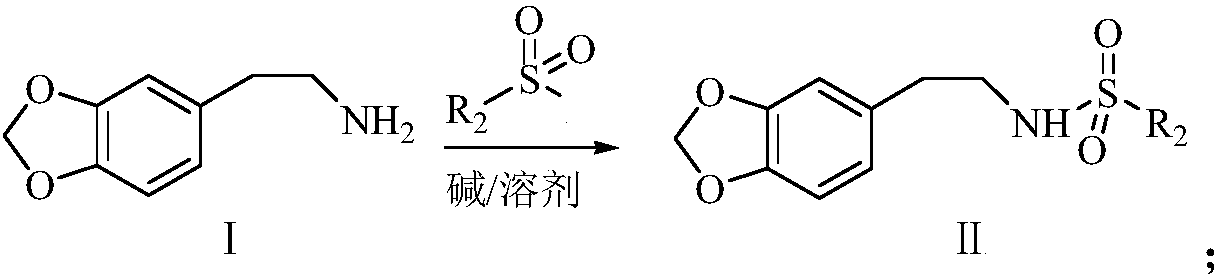
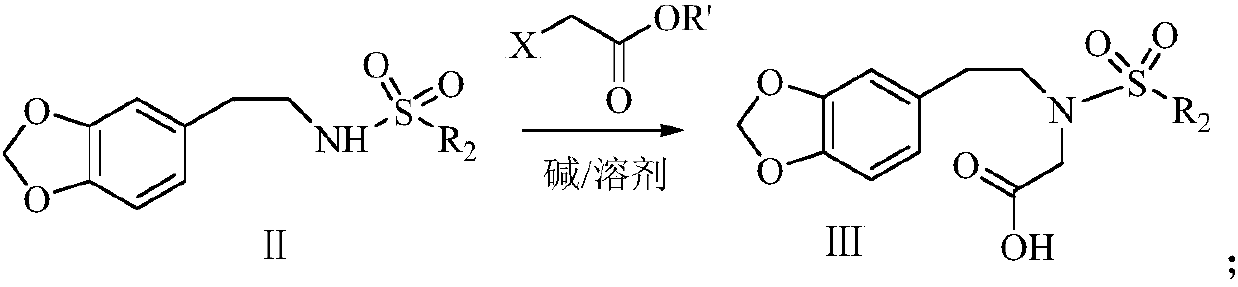
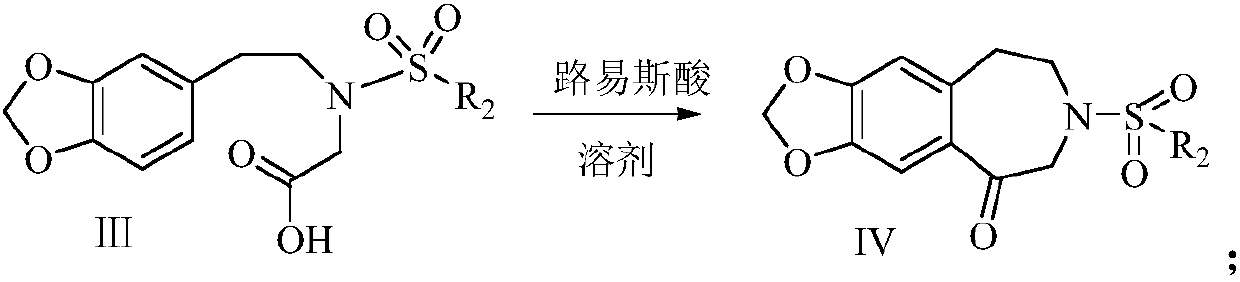
The synthetic method of harringtonine C ring intermediate
A technology of harringtonine and intermediates, which is applied in the field of synthesis of pharmaceutical intermediates, can solve the problems of expensive raw materials, environmental pollution, long routes and the like, and achieves the effect of simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Preparation of N-p-nitrobenzenesulfonyl-3,4-methyleneoxybenzo-3-N-heteroheptanone from 3,4-methylenedioxyphenethylamine
[0041] The first step: amino protection and N-H bond activation: put the flask containing 1.652g (10mmol) 3,4-methylenedioxyphenethylamine in 80mL dichloromethane solution in an ice bath, and then add triethylamine 12mmol to provide an alkaline environment. While stirring, 20 mmol of p-nitrobenzenesulfonyl chloride was slowly added dropwise. After 0.5 h, the ice bath was removed, and the reaction was continued for 4 h at room temperature, and the reaction was stopped. The reaction solution was washed twice with water and once with saturated sodium chloride, and then dried over anhydrous sodium sulfate. Filtration, rotary evaporation to recover the solvent, add petroleum ether to the remaining red oily liquid, after the crystallization is complete, recrystallize to obtain a light yellow solid product N-p-nitrobenzenesulfonyl-3,4-methylenedioxyphenet...
Embodiment 2
[0048] Preparation of N-trifluoromethylsulfonyl-3,4-methyleneoxybenzo-3-N-hepanone from 3,4-methylenedioxyphenethylamine
[0049] Step 1: Amino protection and N-H bond activation: Place a flask containing 1.652g (10mmol) of 3,4-methylenedioxyphenethylamine in 80mL of chloroform solution in an ice bath, then add diisopropylethylamine Amine 11mmol to provide an alkaline environment. While stirring, 18 mmol of trifluorosulfonic anhydride was slowly added dropwise. After 1 h, the ice bath was removed, and the reaction was continued for 3 h at room temperature, and the reaction was stopped. The reaction solution was washed with water three times and saturated sodium chloride once, and dried over anhydrous sodium sulfate. Filtration, rotary evaporation to recover the solvent, adding hexane to the remaining oily liquid to crystallize, after the crystallization is complete, recrystallize to obtain the light yellow solid product N-trifluoromethylsulfonyl-3,4-methylenedioxyphenethylam...
Embodiment 3
[0059] Preparation of N-tert-butoxycarbonyl-3,4-methyleneoxybenzo-3-N-heptanone from 3,4-methylenedioxyphenethylamine
[0060] The first step: amino protection and N-H bond activation: put the flask containing 1.652g (10mmol) 3,4-methylenedioxyphenethylamine in 15mL ethanol solution (ethanol as solvent) in an ice bath, add 10mmol Triethylamine and 3 mmol DBU (1,8-diazacyclo[5,4,0]undecene-7) to provide a basic environment. While stirring, 20 mmol of di-tert-butyl carbonate anhydride was slowly added dropwise to the system, and the ice bath was removed 0.5 h after the dropwise addition, and the reaction was continued for 5 h at room temperature. After the reaction was completed, it was concentrated, and 150 mL of ethyl acetate was added to the residue, washed once with water and once with saturated sodium chloride, and dried over anhydrous sodium sulfate. Filtrate with suction, concentrate the filtrate, and recrystallize from ethyl acetate-petroleum ether to obtain N-tert-buto...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com