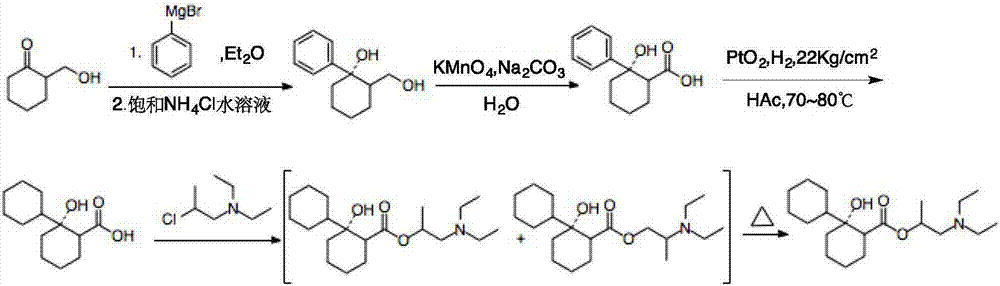
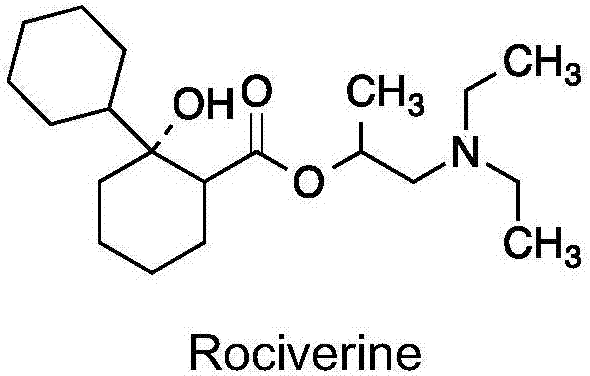
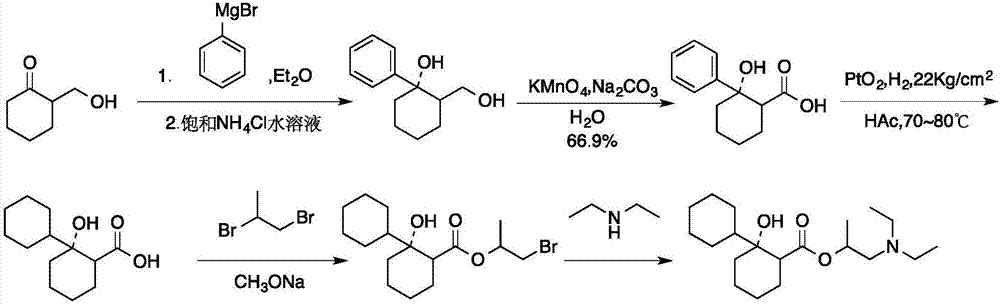
Preparation method of 2-diethylamino-1-methylethyl-7-cyclohexyl-7-oxoheptanoate
A technology of methylethyl and diethylamino, which is applied in the field of chemical drug synthesis, can solve the problems of low content and inability to obtain, and achieves the effects of simple processing and convenient and easy-to-obtain products.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach
[0038] step 1) , cyclic ester 4 Preparation of:
[0039] First add m-chloroperoxybenzoic acid into the reaction flask, then add the solvent dichloromethane, stir in an ice bath to cool down, drop to -5°C~5°C, add cycloheptanone dropwise, after the dropwise addition, heat up to 25°C~ 40°C, continue to stir for 5-10 days, after the reaction, add saturated sodium thiosulfate aqueous solution to quench, after quenching, wash with saturated sodium bicarbonate aqueous solution several times, then wash with saturated brine, and dry over anhydrous magnesium sulfate , concentrated to obtain the crude compound 4, which was passed through the column to obtain compound 4. In step 1), the reaction temperature is preferably 25°C (room temperature), and the reaction time is preferably 7 days.
[0040] step 2) ,alcohol 7 Preparation of:
[0041]Add compound 4, N,O-dimethylhydroxylamine hydrochloride, and dichloromethane as a solvent to the reaction flask in sequence, cool down...
Embodiment 1
[0049] Embodiment one: the preparation of cyclic ester 4
[0050] First add 85% m-CPBA (95g, 0.55mol, 1.8eq) and dichloromethane (1000ml) into a 2L reaction flask in turn, cool down in an ice bath and stir, and when the temperature of the reaction system reaches 0°C, start to add cyclic Heptanone (34g, 0.31mol), the temperature of the system remained basically unchanged during the dropwise addition, the ice bath was removed after the dropwise addition, and the stirring was continued at room temperature (25°C), and the reaction was carried out for 7 days, during which the reaction solution changed from transparent to white turbid. Add saturated sodium thiosulfate solution to quench, add 10% sodium bicarbonate aqueous solution to wash several times after quenching, saturated brine, dry the organic layer, filter, concentrate to obtain compound 4 crude product, and then obtain compound 4 through column chromatography ( 19.5g), yield 50.2%. [M+H] + =129
[0051] 1 H-NMR (400MHz...
Embodiment 2
[0052] Embodiment two: the preparation of alcohol 7
[0053] Add compound 4 (0.64g, 5mmol), N, O-dimethylhydroxylamine hydrochloride (0.4875g, 5mmol, 1eq), dichloromethane (20ml) into the reaction flask in turn, stir and cool down in an ice-salt bath, when the system When the temperature reached -3~-4°C, 2M THF solution of isopropylmagnesium chloride (5.5ml, 11mmol, 2.2eq) was added dropwise. After the dropwise addition, a golden yellow liquid was obtained, and the stirring was continued at low temperature for 1h. After the completion of the reaction was monitored by pointing the plate, slowly add 1M THF solution of cyclohexane magnesium bromide (15ml, 15mmol, 3eq) dropwise. The temperature of the system was kept at -3.5°C. Aqueous solution (10ml) was quenched, washed twice with saturated aqueous sodium bicarbonate solution, washed twice with water, the organic layer was dried, filtered, concentrated to give 1.72g of yellow oil, 0.2g of alcohol 7 was obtained by column chromat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com