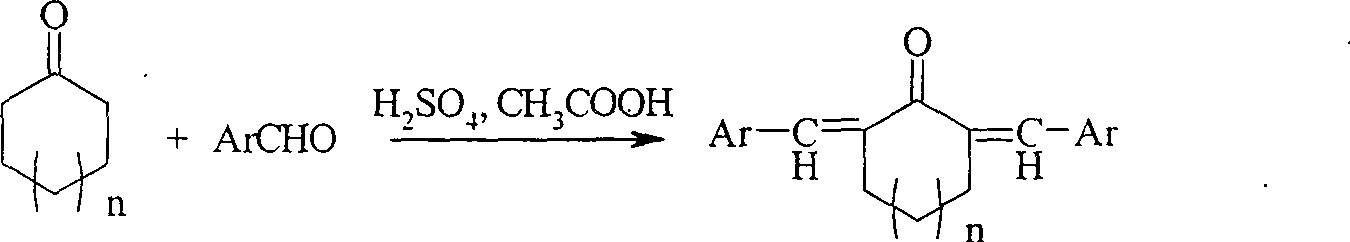Method of preparing alpha,alpha'-di(substituted benzylidene)cyclone ultraviolet radiation absorbent
An ultraviolet and absorber technology, applied in the field of ultraviolet absorbers, can solve the problem that the sunscreen effect is not very high, and achieve the effect of stable chemical properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
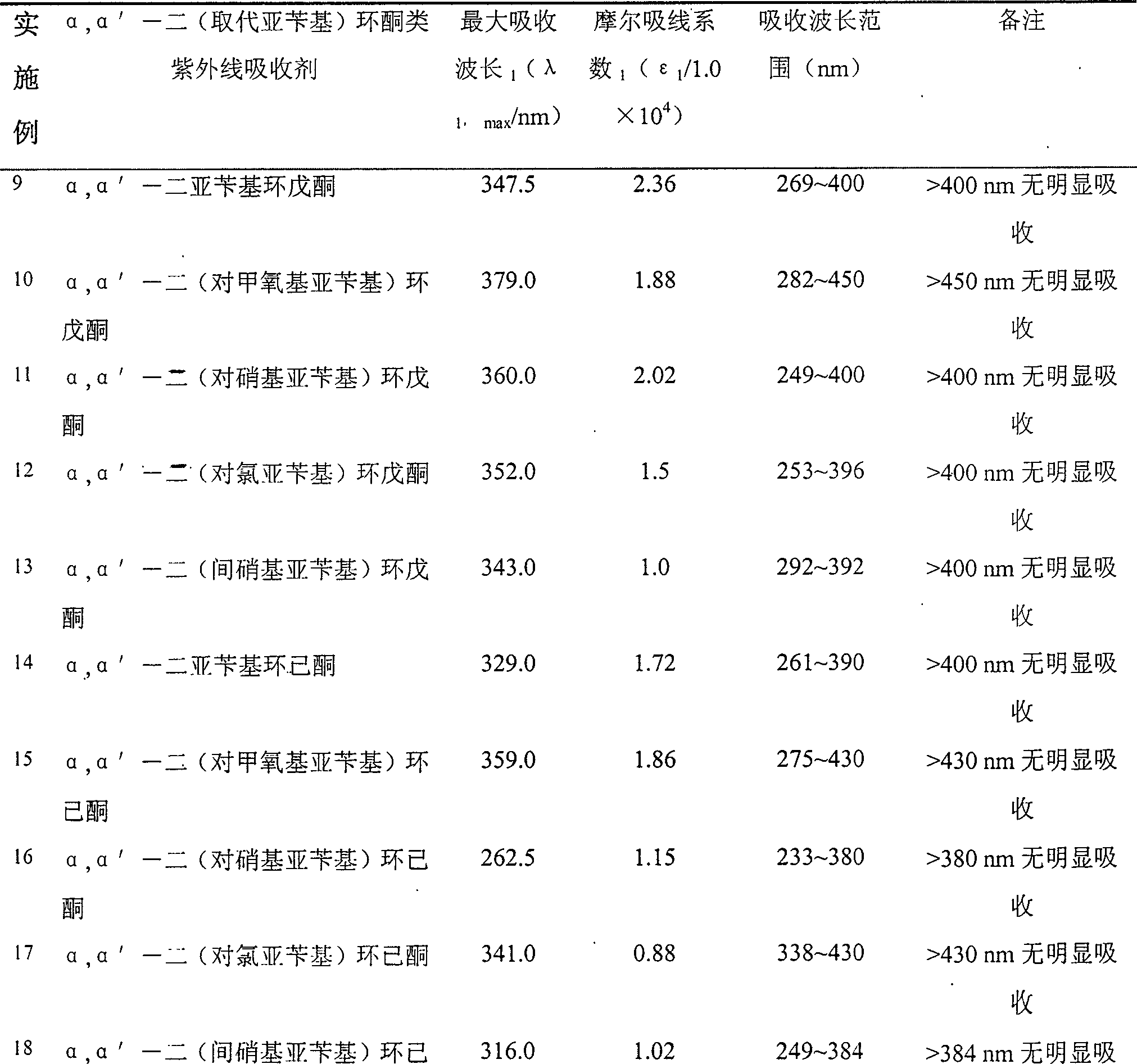
Image
Examples
Embodiment 1
[0025] Add 0.5ml of concentrated sulfuric acid and 7ml of acetic acid into a 100ml three-necked flask (the volume ratio of concentrated sulfuric acid and acetic acid is 1:14), when the mixture of concentrated sulfuric acid and acetic acid is cooled to room temperature (20-25°C), slowly add Cyclopentanone or cyclohexanone and aromatic aldehyde (benzaldehyde, p-methoxybenzaldehyde, p-nitrobenzaldehyde, m-nitrobenzaldehyde, p-chlorobenzaldehyde) mixed solution, cycloketone and aromatic aldehyde in the mixed solution The molar ratio of aldehydes is 1:2, and the concentration of cyclic ketones in the mixed solution of concentrated sulfuric acid and acetic acid is 2.67 (mol / liter). After stirring at room temperature for about 7 hours, a large amount of precipitation is formed. Add 40-60ml of After washing with water, the solid was filtered off with suction, washed with distilled water, and then rinsed with aqueous methanol (the volume ratio of methanol:water was 1:1). The resulting ...
Embodiment 2
[0027] Add 2ml of concentrated sulfuric acid and 10ml of acetic acid (the volume ratio of concentrated sulfuric acid to acetic acid is 1:5) in a 100ml three-necked flask, control the reaction temperature at 35°C, slowly add cycloheptanone and aromatic aldehyde (benzaldehyde, p-formaldehyde) dropwise Oxybenzaldehyde, p-nitrobenzaldehyde) mixed solution, the molar ratio of ketone and aromatic aldehyde in the mixed solution is 1:3, and the concentration of cyclic ketones in the mixed solution of concentrated sulfuric acid and acetic acid is 1.67 (mol / liter) , after stirring at 35°C for about 20 hours, add 50% sodium hydroxide solution to neutralize the reaction solution to neutrality, let the reaction solution stand for 3 to 4 hours, a large amount of precipitation is formed, filter out the solid, and use 5 to Wash with 10 ml ethyl acetate. The resulting solid was recrystallized from dichloromethane and ethanol, respectively. To obtain the corresponding product α, α'-dibenzylide...
Embodiment 3-8
[0029] Influence of Concentrated Sulfuric Amount in the Reaction of Cyclohexanone and Benzaldehyde
[0030]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com