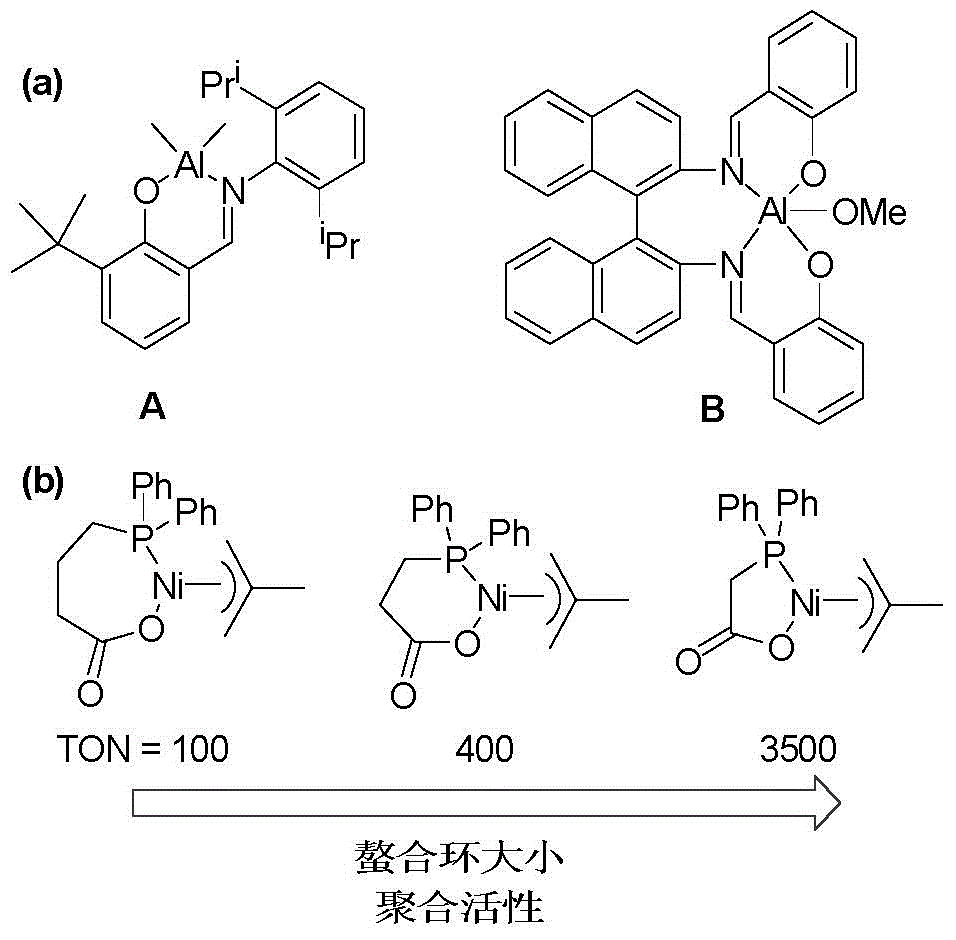
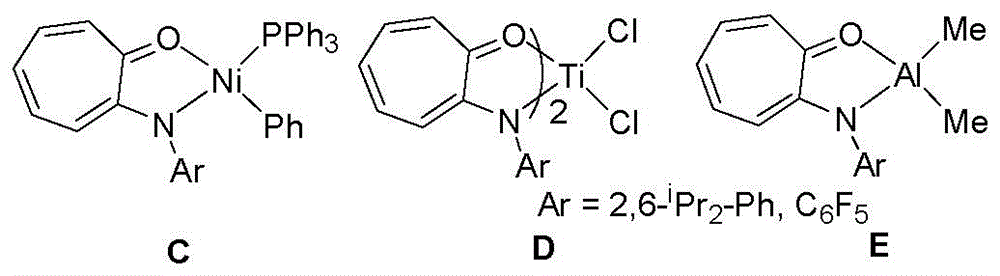
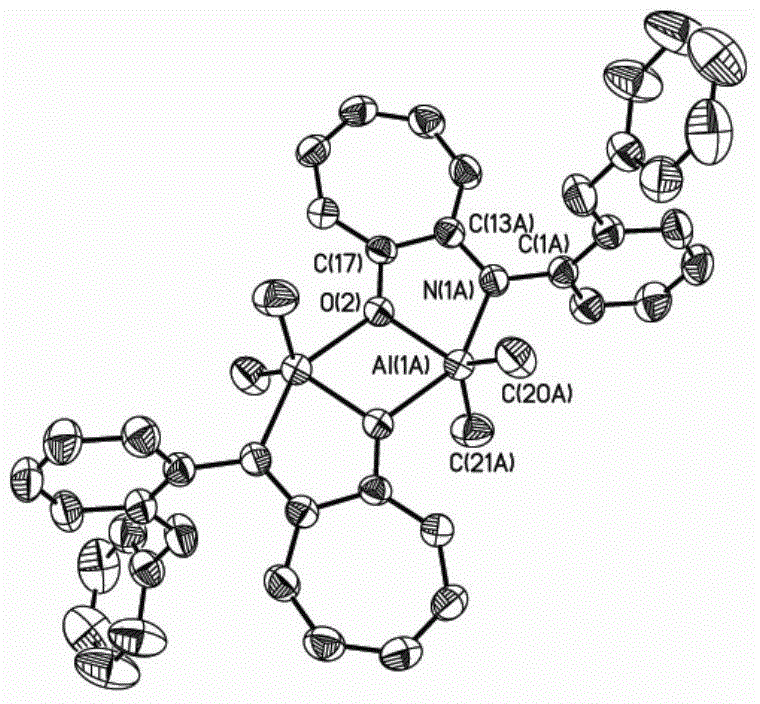
Cycloheptatriene-structure-containing aluminium compound catalysts, synthesis thereof and uses of the catalysts
A technology of cycloheptatriene and cycloheptatrienone is applied in the field of aluminum compound catalyst containing cycloheptatriene structure, synthesis and application thereof, and can solve the problems of limited application, low activity of aluminum-containing catalyst and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Synthesis of 2-[2-(phenoxy)anilino]tropone
[0039]
[0040] Under nitrogen, add tris(dibenzylidene-BASE acetone)dipalladium (0.09 g, 0.01 mmol, purchased from Anaiji Company), 1,1'-binaphthyl-2, 2'-bisdiphenylphosphine (0.012 g, 2.0 mmol, purchased from Anaiji Company), 2-trifluoromethanesulfonate cycloheptatrienone (0.53 g, 2.0 mmol), 2-phenoxy Aniline (0.44 g, 2.4 mmol, purchased from Anaji) and 4 mL of toluene. The mixture was stirred at 80° C. for 24 hours, cooled down to room temperature after the reaction, filtered through a 10 cm silica gel column, and washed with 200 ml of dichloromethane. Finally, the eluent was passed through the column with petroleum ether:ethyl acetate=10:1 to obtain a yellow product (0.48 g, yield 83%). 1 HNMR (400MHz, CDCl 3 ):δ=8.75(s,1H,NH),7.52–7.47(m,1H,CH),7.29(d,J=7.3Hz,2H,CH),7.23(d,J=1.1Hz,1H,CH ),7.20–7.17(m,2H,CH),7.14(d,J=4.4Hz,2H,CH),7.07(m,J=9.1,1H,CH),6.93(d,J=7.7Hz,2H ,CH),6.76(d,J=8.3Hz,1H,CH). 13 CNMR (100MHz, CD...
Embodiment 2
[0042] Synthesis of 2-{2-[(2,6-diisopropyl)phenoxy]anilino}tropone
[0043]
[0044] Add tris(dibenzylidene-BASEacetone)dipalladium (0.09 g, 0.01 mmol), 1,1'-binaphthyl-2,2'-bisdiphenylphosphine to a Schlenk bottle with a magnet under nitrogen (0.012 g, 2.0 mmol), 2-trifluoromethanesulfonate cycloheptatrienone (0.53 g, 2.0 mmol), 2-[(2,6-diisopropyl)phenyl]aniline (0.65 g, 2.4 mmol) and 4 ml of toluene. The mixture was stirred at 80° C. for 24 hours, cooled down to room temperature after the reaction, filtered through a 10 cm silica gel column, and washed with 200 ml of dichloromethane. Finally, the eluent was passed through the column with petroleum ether:ethyl acetate=10:1 to obtain a yellow product (0.45 g, yield 60%). 1 HNMR (400MHz, CDCl 3 ):δ=8.99(s,1H,NH),7.53–7.45(m,1H,CH),7.38–7.25(m,2H,CH),7.25–7.14(m,5H,CH),7.08–7.00( m,1H,CH),6.78(m1H,CH),6.52–6.44(m,1H,CH),2.94(m,2H,CH(CH 3 ) 2 ), 1.10 (dd, J=13.8, 6.9Hz, 4H, CH 3 ) ppm. 13 CNMR (100MHz, CDCl 3 ): δ=17...
Embodiment 3
[0046] Synthesis of 2-[2-(phenylmercapto)anilino]tropone
[0047]
[0048] Add tris(dibenzylidene-BASEacetone)dipalladium (0.09 g, 0.01 mmol), 1,1'-binaphthyl-2,2'-bisdiphenylphosphine to a Schlenk bottle with a magnet under nitrogen (0.012 g, 2.0 mmol), 2-trifluoromethanesulfonate tropotrienone (0.53 g, 2.0 mmol), 2-aminodiphenyl sulfide (0.44 g, 2.4 mmol, from Anaiji company) and 4 ml of toluene. The mixture was stirred at 80° C. for 24 hours, cooled down to room temperature after the reaction, filtered through a 10 cm silica gel column, and washed with 200 ml of dichloromethane. Finally, the eluent was passed through the column with petroleum ether:ethyl acetate=10:1 to obtain a yellow product (0.48 g, yield 83%). 1 HNMR (400MHz, CDCl 3 ): δ=8.86(s,1H,NH),7.42(s,2H,CH),7.46–7.13(m,9H,CH),7.06(t,J=10.2Hz,1H,CH),6.96(d ,J=10.3Hz,1H,CH),6.74(t,J=9.1Hz,1H,CH)ppm. 13 CNMR (100MHz, CDCl 3 ):δ=177.23(C=O),152.97(C-N),138.25,137.37,135.72,134.13,133.52,131.93,131.46(,131.1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com