Synthesis method of poly-substituted oxacycloheptatriene-3(2H) ketone compounds
A technology for oxepatriene and ketone compounds, which is applied in the field of synthesis of multi-substituted oxepatriene-3-one compounds, and can solve the problems of difficult preparation of starting materials, harsh reaction conditions, troublesome operation, etc. problems, to achieve the effect of convenient preparation, simple reaction conditions, and easy separation and purification
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0016]
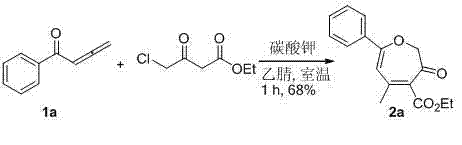
[0017] In a 10 mL round bottom flask was charged 1a (1 mmol, 144 mg), ethyl 4-chloroacetoacetate (1.2 mmol, 197.5 mg) and 3 mL of acetonitrile, followed by potassium carbonate (1 mmol, 138 mg). After stirring at room temperature for 1 hour, the reaction was quenched by adding 2 mL of saturated ammonium chloride solution, extracted with ethyl acetate (5 mL×3), washed with saturated brine, and dried over anhydrous sodium sulfate. Filter, spin dry, and separate through silica gel column (petroleum ether / ethyl acetate = 10 / 1) to obtain the yellow solid product 5-methyl-3-oxo-7-phenyl-2,3-dihydrocycloheptatriene - Ethyl 4-carboxylate 2a (185mg, 68%). The characterization data of this compound are as follows: 1 H NMR (400 MHz, CDCl 3 ) δ: 1.35 (t, J = 7.6 Hz, 3H), 2.21 (s, 3H), 4.34 (q, J = 7.6 Hz, 2H), 4.69 (s, 2H), 6.02 (s, 1H), 7.40-7.46 (m, 3H), 7.71 (d, J = 8.0 Hz, 2H). 13 C NMR (100 MHz, CDCl 3 ) δ: 14.15, 24.82, 61.46, 77.46, 106.94, 127.11, 128.72, 131....
Embodiment 2
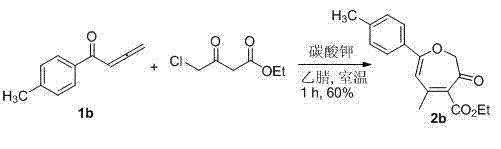
[0019] According to the method described in Example 1, add 1a (1 mmol, 144 mg), ethyl 4-chloroacetoacetate (1.2 mmol, 197.5 mg) and 3 mL of acetone into a 10 mL round bottom flask, and then add potassium carbonate (1 mmol, 138 mg). After stirring at room temperature for 1 hour, the product ethyl 5-methyl-3-oxo-7-phenyl-2,3-dihydrocycloheptatriene-4-carboxylate 2a (163 mg, 60%) was obtained.
Embodiment 3
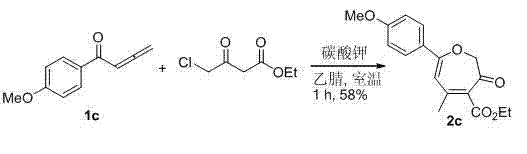
[0021] According to the method described in Example 1, 1a (1 mmol, 144 mg), ethyl 4-chloroacetoacetate (1.2 mmol, 197.5 mg) and 3 mL of dimethyl sulfoxide were added to a 10 mL round bottom flask, and then Potassium carbonate (1 mmol, 138 mg) was added. After stirring at room temperature for 1 hour, the product ethyl 5-methyl-3-oxo-7-phenyl-2,3-dihydrocycloheptatriene-4-carboxylate 2a (201 mg, 74%) was obtained.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com