3-methyl-octahydro-cycloheptatriene oxapicene-2-ketone and preparation method thereof
A technology of cycloheptatriene and cycloheptanone, which is applied in the field of 3-methyl-octahydrocycloheptatrien-2-one and its preparation, can solve the complicated and troublesome synthesis method and has no industrial production value and other issues, to achieve the effect of cheap raw materials, low cost, and easy availability of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
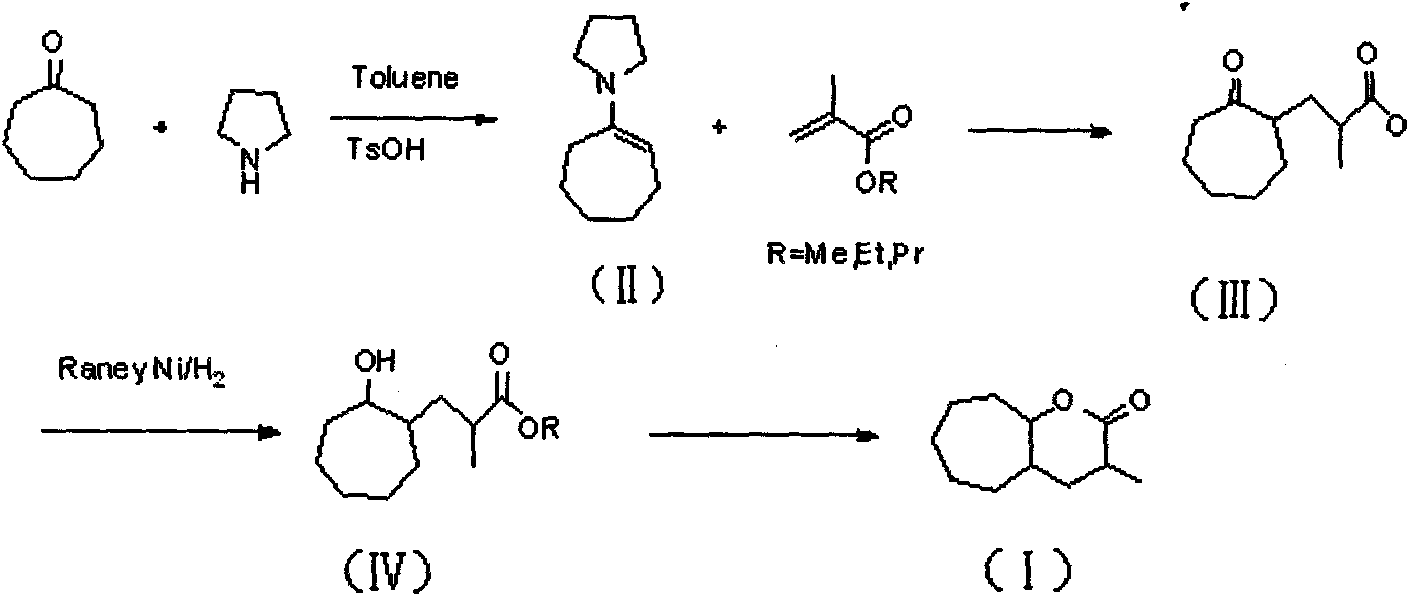
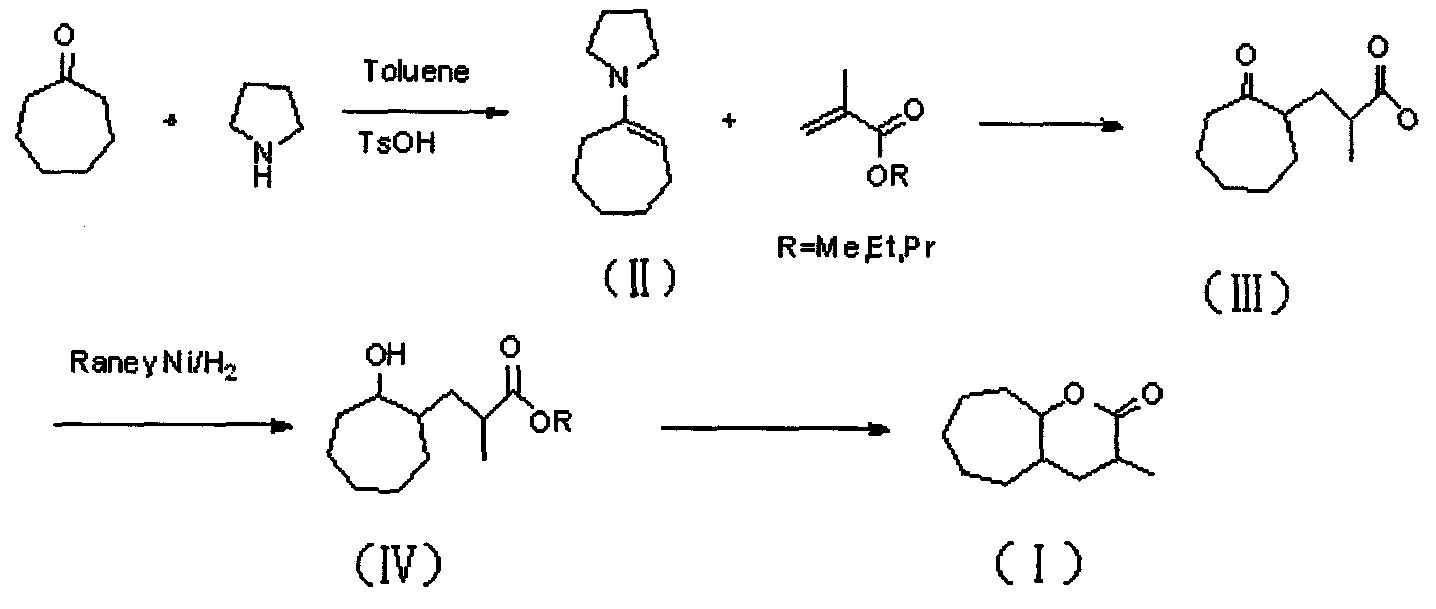
[0050] A preparation method of 3-methyl-octahydrocycloheptatrien-2-one, comprising the following preparation steps:
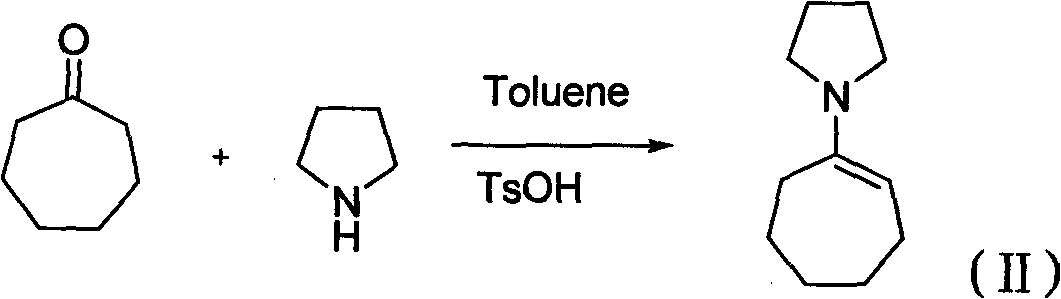
[0051] (1), cycloheptanone and pyrrolidine are added in solvent, with the method for acid catalysis, carry out dehydration reaction, obtain the cycloheptylpyrrole enamine of structural general formula (II);
[0052] (2), cycloheptylpyrrole enamine and methacrylic acid ester with the general structure formula (II) are heated to reflux in a solvent to carry out michael addition reaction, and then the reaction mixture is hydrolyzed with an acid treatment, heated to reflux, and the pyrrolylamine group is removed, Bare ketone group; Reaction obtains the ester derivative of the cycloheptanone of structural formula (III), namely 2-methyl propionate-cycloheptanone;
[0053] (3), 2-methylpropionate-cycloheptanone with the general structural formula (III) is added in the autoclave, a solvent and a hydrogenation catalyst are added; hydrogen at a certain pressure is charge...
Embodiment 1
[0058] Preparation of 3-methyl-octahydrocycloheptatrien-2-one
[0059] (1), the preparation of cycloheptyl pyrrole enamine
[0060] In a 500mL three-necked flask equipped with a thermometer and an oil-water separator, add 58g of cycloheptanone, 70mL of toluene, 63g of pyrrolidine, and 1g of p-toluenesulfonic acid. The reaction system was heated under reflux for 4 hours. When 9.36 g of water was obtained, the reaction was stopped, cooled to room temperature, 1.1 g of sodium acetate was added, and the reaction solution was filtered. The filtrate was evaporated to remove the solvent under reduced pressure. 57g of the fraction at 135-146°C / 133pa is the product with a yield of 66% and a purity of 91% by GC analysis.
[0061] (2), the preparation of 2-methylpropionate-cycloheptanone
[0062] Add 53g of cycloheptylpyrrolene amine and 100mL of methanol into a 250mL three-necked flask, heat to reflux, add 40g of methyl methacrylate dropwise within 1h, continue to reflux for 3h after ...
Embodiment 2
[0068] Preparation of 3-methyl-octahydrocycloheptatrien-2-one
[0069] (1), the preparation of cycloheptyl pyrrole enamine
[0070] In a 500mL three-necked flask equipped with a thermometer and an oil-water separator, add 58g of cycloheptanone, 70mL of cyclohexane, 63g of pyrrolidine, and 1g of p-toluenesulfonic acid. The reaction system was heated under reflux for 5 hours. When 9.36 g of water was obtained, the reaction was stopped, cooled to room temperature, 1.2 g of sodium acetate was added, and the reaction solution was filtered. The filtrate was evaporated to remove the solvent under reduced pressure. 60g of the fraction at 140-1148°C / 133pa is the product with a yield of 70% and a purity of 92% by GC analysis.
[0071] (2), the preparation of 2-methylpropionate-cycloheptanone
[0072] Add 53g of cycloheptylpyrrolene amine and 100mL of ethanol to a 250mL three-necked flask, heat to reflux, add 40g of methyl methacrylate dropwise within 1h, continue to reflux for 3h afte...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com