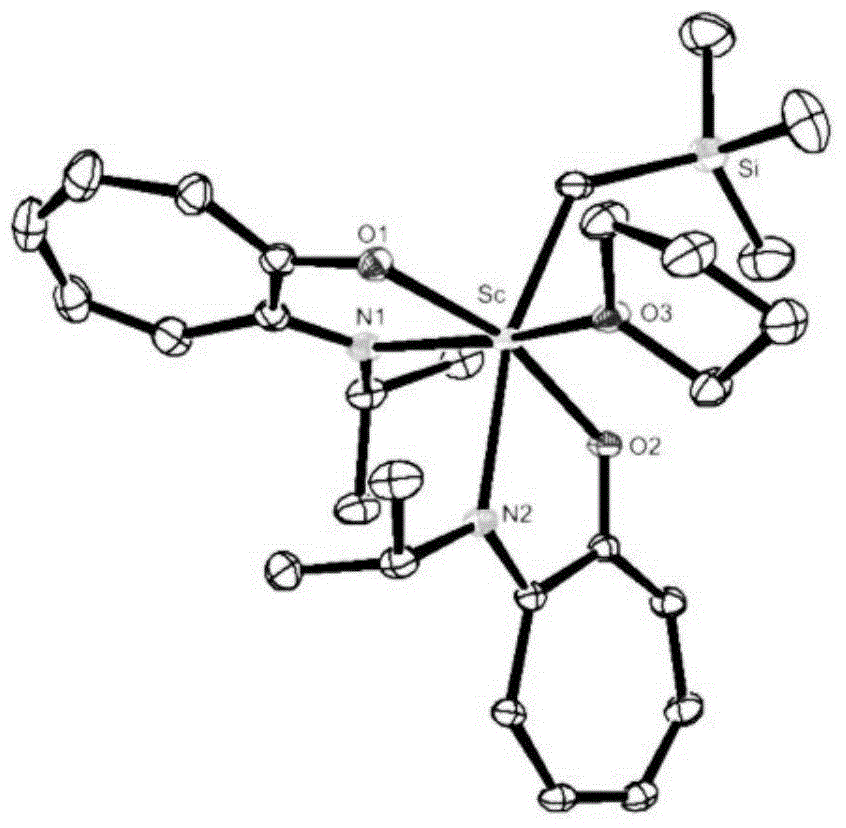
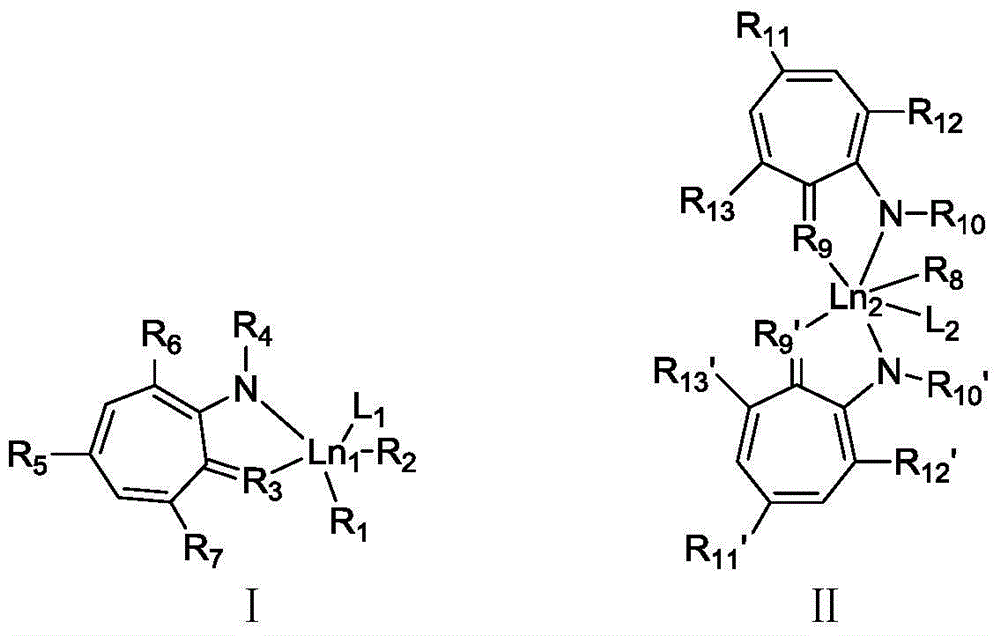
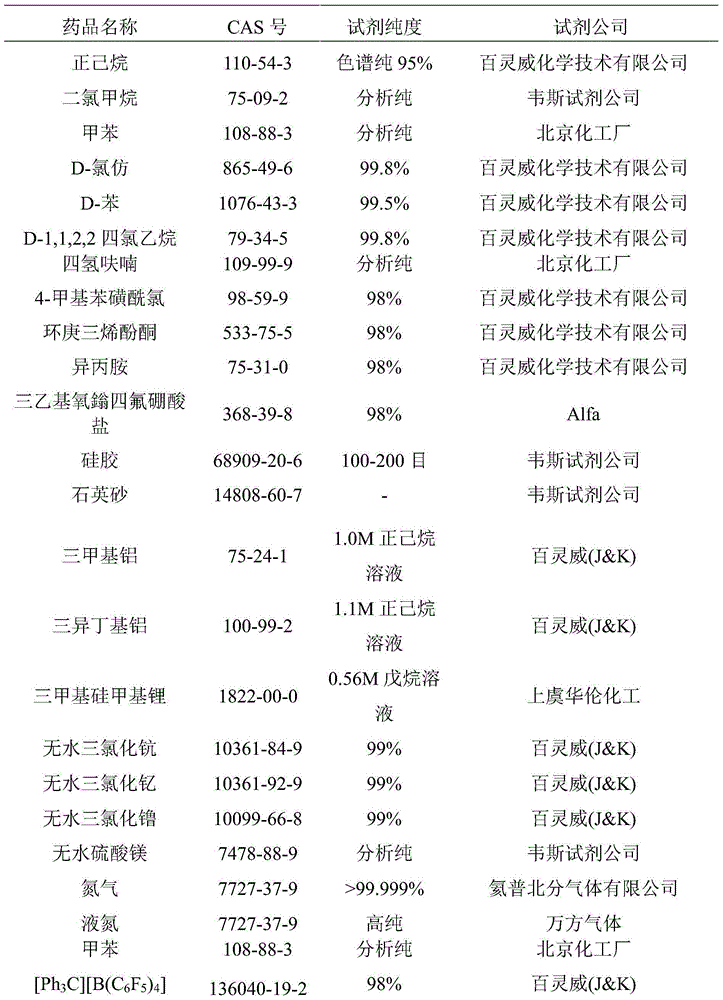
Cycloheptatriene-base rare-earth metal catalyst, and preparation method and application thereof
A technology of cycloheptatrienyl rare earth and metal catalysts, which is applied in chemical instruments and methods, organic chemistry, and compounds containing elements of Group 3/13 of the periodic table, etc. Reporting, long response time, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0073] (1) Preparation of cycloheptatrienyl ligand
[0074]
[0075] First, weigh 5.3 g (43 mmol) of tropolone and 8.2 g (43 mmol) of p-toluenesulfonyl chloride into the reactor, add 60 mL of dichloromethane at room temperature, start stirring the mixture, and then add 6 mL of (43mmol) triethylamine, this moment will produce yellow muddy substance. Another 60 mL of dichloromethane was added. Compound a was obtained after stirring for about 32 h at room temperature under an atmosphere of nitrogen protection.
[0076]Next, isopropylamine (60 mL) was slowly added dropwise into a (10.92 g, 40.0 mmol) at low temperature. After the mixture was stirred overnight at room temperature, it was rotary evaporated under reduced pressure, and the obtained substance was washed with 2mol / L NaOH (60mL) and CH 2 Cl 2 (120 mL) was dissolved, the layers were separated and the organic phase was separated. CH 2 Cl 2 (120 mL) was extracted twice. The organic phases were combined, washed wi...
Embodiment 2
[0083] (1) Preparation of cycloheptatrienyl ligand
[0084]
[0085] First, weigh 5.3 g (43 mmol) of tropolone and 8.2 g (43 mmol) of p-toluenesulfonyl chloride into the reactor, add 60 mL of dichloromethane at room temperature, and then add 4.4 g (6 mL, 43mmol) triethylamine, and another 60mL of dichloromethane was added. Under the protection of nitrogen at room temperature, after stirring for about 32 h, compound a was obtained. The operation of compound b is the same as in Example 1. Second, the CH of b product (1.37g, 9.20mmol) 2 Cl 2 The solution was slowly added to the Et 3 OBF 4 (1.75g, 9.21mmol) solution, after stirring at room temperature for 3h, spot the plate until the point of the new product no longer changes, then cool the reaction to 0°C, and then slowly add isopropylamine (20mL) dropwise to the above In the reaction flask, after the reaction returned to room temperature, it was stirred overnight to obtain a mixture e containing the target product. The...
Embodiment 3
[0092] (1) Preparation of cycloheptatrienyl ligand
[0093]
[0094] The operation of compounds a and b is the same as in Example 1. Afterwards, the CH of b product (1.37g, 9.20mmol) 2 Cl 2 The solution was slowly added to the Et 3 OBF 4 (1.75g, 9.21mmol) solution, after stirring at room temperature for 3h, point the plate, confirm that there is a new product, until the point of the new product does not change, the reaction is cooled to 0 ° C, and then isopropylamine (20mL ) was slowly added dropwise at a rate of 1 s / drop into the reaction flask, and after the reaction returned to room temperature, it was stirred overnight to obtain a mixture e containing the target product. The product was purified by column chromatography to obtain 1.39 g of the cycloheptatrienyl ligand f with a yield of 47.9%.
[0095] (2) Preparation of Cycloheptatrienyl Lutetium Catalyst
[0096]
[0097] First, the reactor was placed in a glove box, and the cycloheptatrienyl ligand f (326.9 m...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com