Application of 1,3,4,5-tetrahydro-2-benzo-cycloheptatriene compound to in vitro screening or drug preparation
A technology of dibenzoxepin and benzoxepatriene, which is applied in the field of traditional Chinese medicine, can solve the problems of restricting the scientific use of medicinal plants and hindering the wide application of pinnatifida, and achieve the protection of cardiomyocytes Injury, reduce the effect of cardiomyocyte injury
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
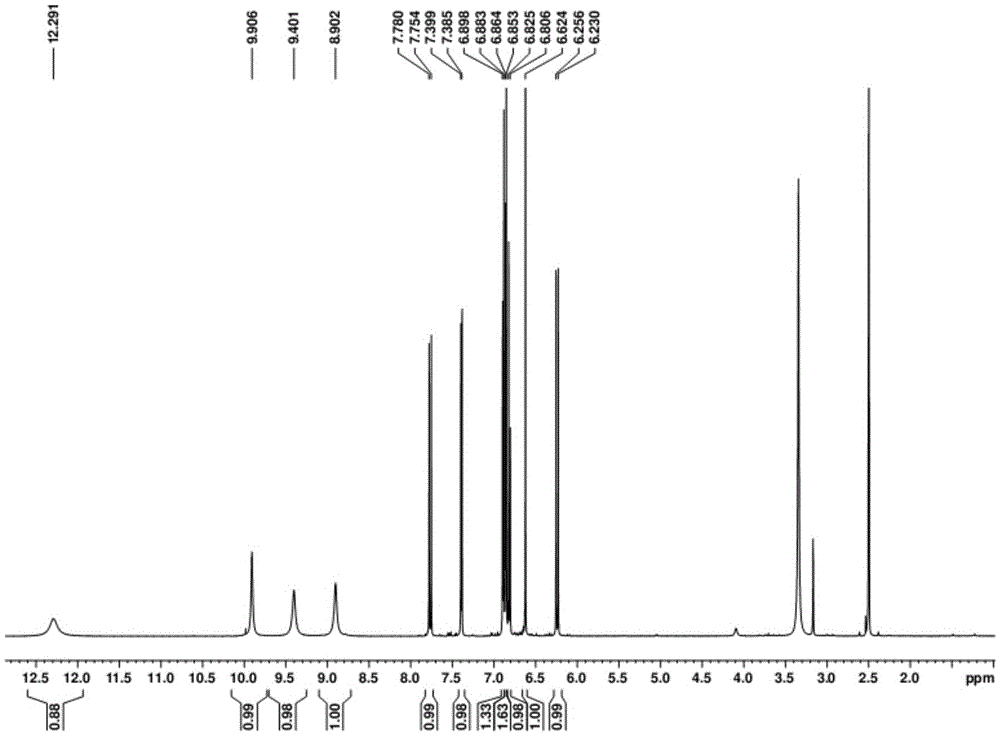
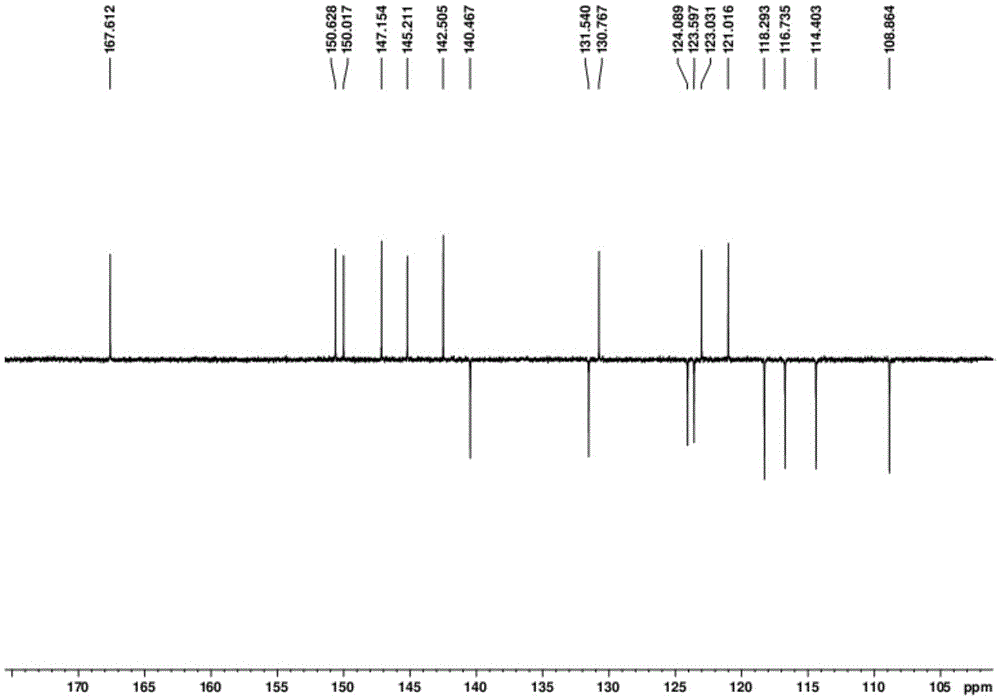
[0034]In a typical implementation of the present invention, the preparation method of the above-mentioned dibenzoxepin-based compounds comprises the following steps: using a solvent to reflux extract the aerial part of Campanula chinensis to obtain an extract; The first extraction solvent extracts the extract for the first time to obtain the first extract; preferably the first extraction solvent is selected from petroleum ether, dichloromethane or chloroform; the second extraction solvent is used to extract the petroleum ether extract for the second time , to obtain the second extract; preferably the second extraction solvent is selected from ethyl acetate or n-butanol; the second extract is subjected to silica gel column chromatography, and chloroform is used in the chromatographic process: Methanol is used for gradient washing of the second extract In the gradient elution process, the volume ratio of chloroform to methanol ranges from 100:0, 100:3 to 9 and 100:12 to 30; the e...
Embodiment 1
[0050] The preparation of embodiment 1 compound of the present invention
[0051]After crushing 15kg of the above-ground part of the spinach, extract twice with 70% ethanol under reflux, and recycle the ethanol to obtain about 1.8kg of extract. After dissolving the extract in 6000ml of water, extract three times with 6000ml of petroleum ether, 2000ml each time, recover the solvent under reduced pressure to obtain petroleum ether extract (FS, 100g); extract three times with 18000ml ethyl acetate, 6000ml each time, reduce The solvent was recovered under pressure to obtain an ethyl acetate extract (FY, 700 g), which was extracted three times with 18000 ml of n-butanol, 6000 ml each time, and the solvent was recovered under reduced pressure. Ethyl acetate extract (FY, 700g) was subjected to silica gel column chromatography with chloroform:methanol (100:0~100:100) for gradient elution, and was divided into FY-a (100:0, 5g), FY-b ( 100:3, 25g), FY-c(100:6, 50g), FY-d(100:9, 50g), F...
Embodiment 2
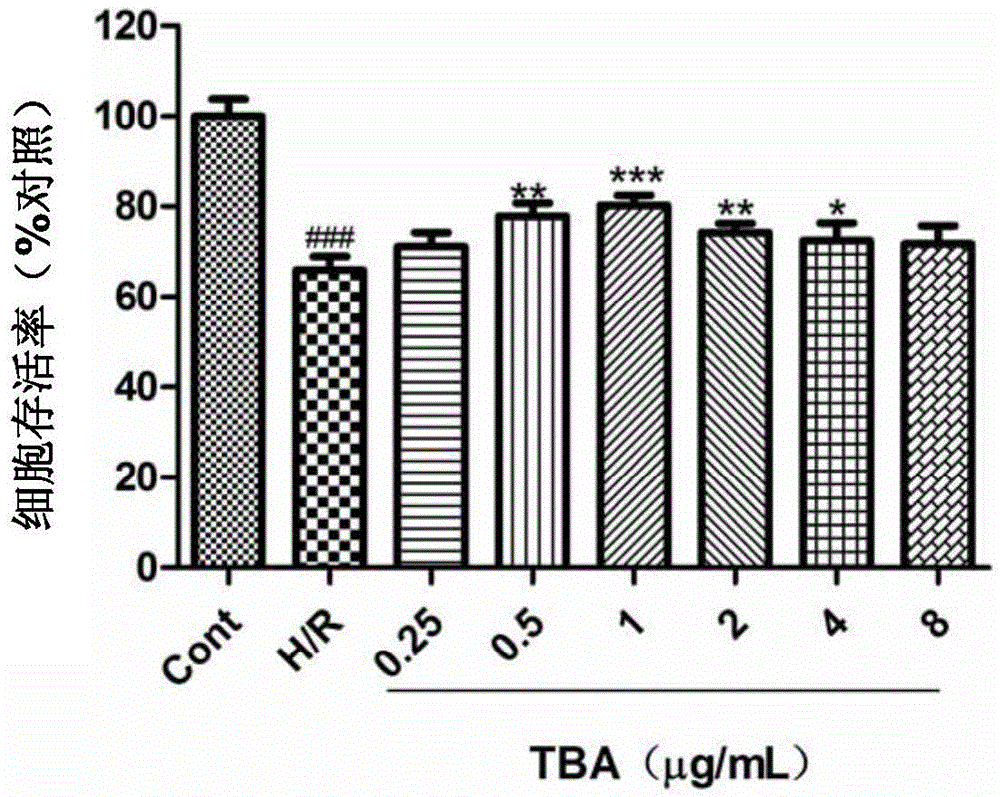
[0057] Example 2 Protective effect of viridescent acid B on H9c2 cardiomyocyte injury induced by hypoxia-reoxygenation
[0058] The experiment was divided into blank control group, hypoxia-reoxygenation model group and viridanic acid B pretreatment group. Among them, the blank control group was treated with serum-free culture medium during the experiment. The hypoxia-reoxygenation model group was cultured in serum-free medium for 2 hours, and then added with a final concentration of 200 μM hypoxia-reoxygenation for 2 hours. In the main active ingredient pretreatment group, the serum-free culture solution containing various active ingredients at the corresponding concentration was treated for 4 hours, the drug-containing culture solution was discarded, and a final concentration of 200 μM was added for hypoxia-reoxygenation for 2 hours.
[0059] Cells in the logarithmic growth phase were divided into 5×10 4 The density of cells / well was seeded in 96-well plate in 5v% CO 2 1. ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com