Polysubstituted difuro-cycloheptatrienamine derivative and preparation method thereof
A technology of cycloheptatrienylamine and difuran, which is applied in the field of multi-substituted bisfuranotropenylamine derivatives and its preparation, can solve the problems of waste of raw materials, many reaction steps, and low utilization rate of atoms
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] Preparation of multi-substituted bisfurocyclohexatriene amine derivatives 3aa:
[0050]
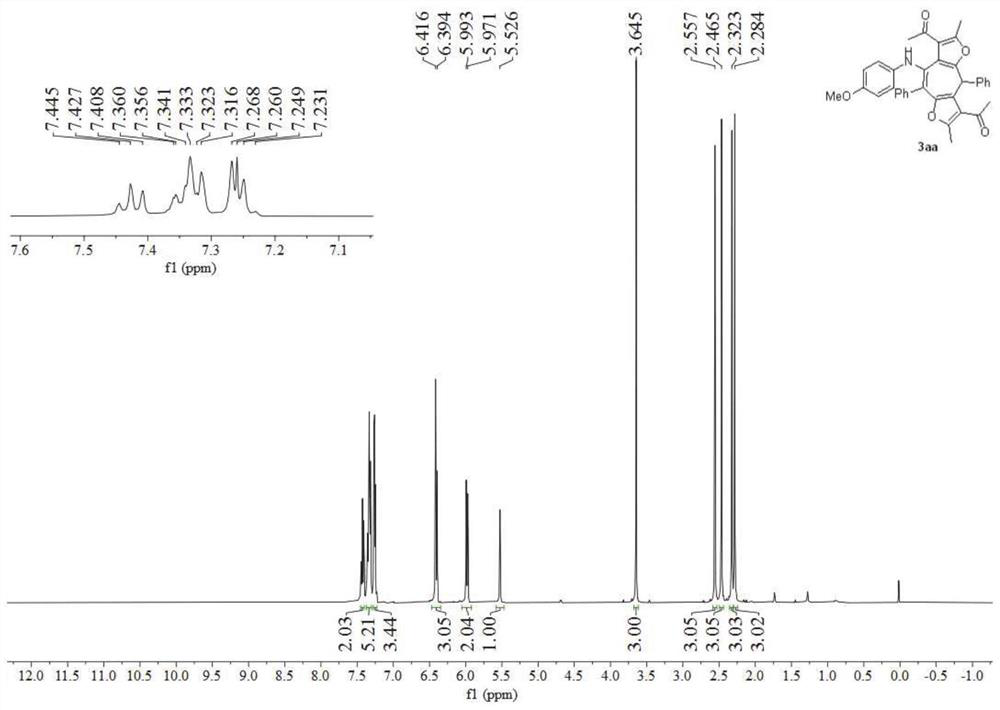
[0051] Add p-methoxyphenylisonitrile 1a (0.3 mmol), 3-(3-phenylpropyl-2-yn-1-ylidene)-2,4-pentanedione 2a (0.6 mmol), and dissolved with dichloromethane (2mL), add a stirring bar, add zinc chloride (0.3mmol), tighten the pressure tube cock and put it into a metal module preheated to 90°C for stirring, the reaction time is 1.5h, At this time, TLC monitored the complete formation of 3aa, stopped the reaction and left it to room temperature, evaporated the solvent under reduced pressure, and separated by silica gel column chromatography to obtain the final product, which was confirmed to be multi-substituted by H NMR, C and mass spectrometry Bisfurocycloheptatriene amine derivative 3aa, the yield is 74%.
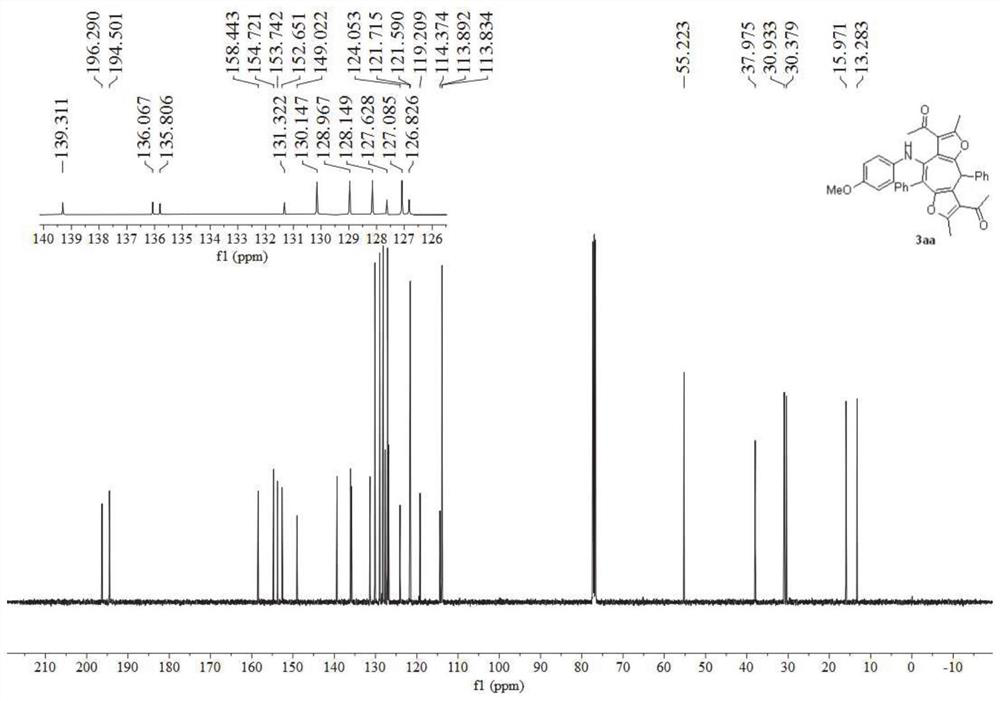
[0052] figure 1 It is the NMR spectrum of the bisfurocycloheptatrienylamine derivative obtained in Example 1 of the present invention, figure 2 Its nuclear magnetic carbon sp...
Embodiment 2
[0055] Preparation of multi-substituted bisfuro cycloheptatriene amine derivatives 3ba:
[0056]
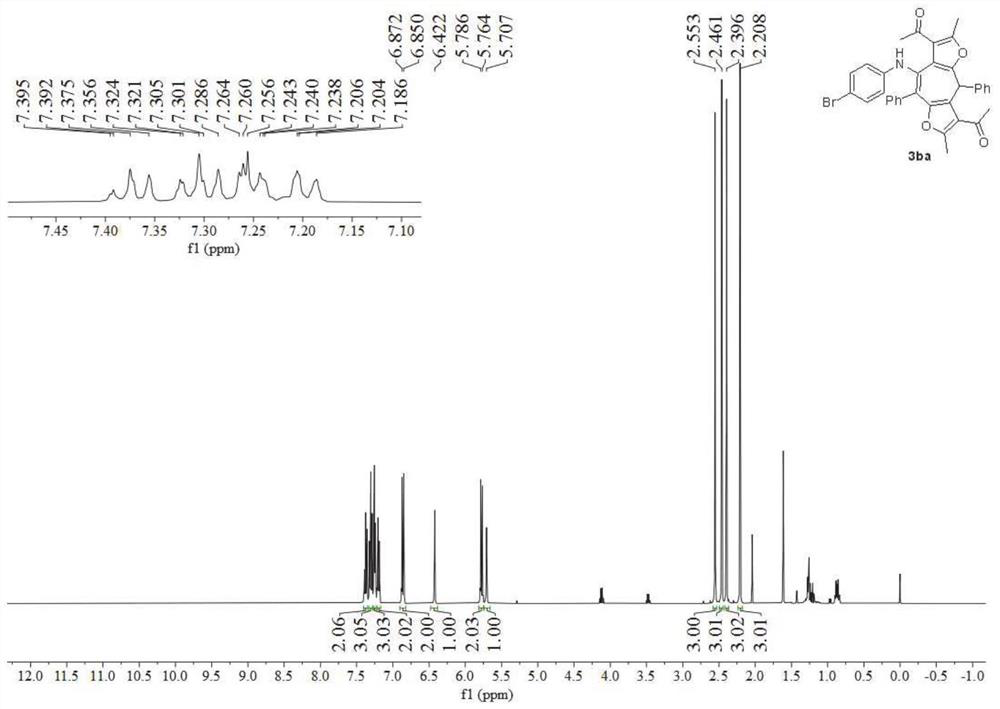
[0057] Use p-bromophenylisonitrile 1b to replace p-methoxyphenylisonitrile 1a in Example 1, and other conditions are the same as in Example 1 to obtain multiple substituted bisfurocycloheptatrienylamine derivatives 3ba with a yield of 65 %.
[0058] image 3 It is the nuclear magnetic hydrogen spectrogram of the bisfurocycloheptatrienylamine derivative obtained in Example 2 of the present invention, Figure 4 Its nuclear magnetic carbon spectrum, spectrum analysis data:
[0059] 1 H NMR (400MHz, CDCl 3 )δ7.38(t, J=7.2Hz, 2H), 7.33–7.28(m, 3H), 7.27–7.23(m, 3H), 7.22–7.17(m, 2H), 6.86(d, J=8.8Hz ,2H),6.42(s,1H),5.78(d,J=8.8Hz,2H),5.71(s,1H),2.55(s,3H),2.46(s,3H),2.40(s,3H) ,2.21(s,3H). 13 C NMR (101MHz, CDCl 3 )δ196.1,194.5,159.0,153.8,153.6,148.6,142.5,139.2,135.7,131.4,129.9,129.3,128.8,128.3,127.7,127.0,123.9,121.8,120.6,119.4,118.5,114.8,112.5,37.9,31.0 ,30.5,16.0,...
Embodiment 3
[0061] Preparation of multi-substituted bisfurocyclohexatriene amine derivatives 3ca:
[0062]
[0063] Use p-ethoxycarbonylphenylisonitrile 1c to replace p-methoxyphenylisonitrile 1a in Example 1, and other conditions are the same as in Example 1 to obtain multi-substituted bisfurocycloheptatrienylamine derivatives 3ca, yield 60%.
[0064] Spectral analysis data:
[0065] 1 H NMR (400MHz, CDCl 3 )δ7.44(d,J=8.8Hz,2H),7.35–7.28(m,4H),7.28–7.23(m,4H),7.22–7.17(m,2H),6.44(s,1H),6.25 (s,1H),5.85(d,J=8.8Hz,2H),4.24(q,J=7.1Hz,2H),2.56(s,3H),2.48(s,3H),2.46(s,3H) ,2.18(s,3H),1.31(t,J=7.1Hz,3H). 13 CNMR (101MHz, CDCl 3 )δ195.9,194.5,166.6,159.3,154.5,153.9,148.5,147.6,139.3,135.8,130.5,129.8,128.5,128.3,128.1,127.7,127.1,127.0,123.8,121.8,121.6,121.3,120.6,115.3,115.1 ,60.2,37.9,31.0,30.5,16.0,14.4,14.3.HRMS(ESI-TOF)m / zcalculated for C 38 h 33 NNaO 6 +
[0066] ([M+Na] + )622.2200, found 622.2196.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com